
Absorbance Plate Readers
Microplate readers and spectrophotometers for visible and UV-visible absorbance detection
SpectraMax Spectrophotometers for UV-Vis Absorbance Detection
The SpectraMax® absorbance spectrophotometers and plate readers provide the versatility and convenience for a wide range of assays such as ELISAs, nucleic acid and protein quantitation, and microbial growth.
Our absorbance plate readers feature our PathCheck Sensor technology and industry-leading SoftMax® Pro Data Acquisition and Analysis Software. They can be combined with our StakMax® Microplate Stacker and can be easily integrated with leading partner robotic systems.
We also provide GxP compliance and validation tools for optimal GLP/GMP lab performance.
Our featured SpectraMax ABS/ABS Plus Microplate Readers
The SpectraMax ABS/ABS Plus Microplate Readers provide fast absorbance detection without the use of filters with monochromator-based wavelength selection for visible and UV-visible absorbance.

EIGHT-CHANNEL OPTICS
Plate reads as fast as five seconds

MICROPLATES OR CUVETTES
Use a 96- or 384-well microplate or a standard cuvette

PATHCHECK SENSOR TECHNOLOGY
Measures the optical pathlength of samples

SoftMax Pro Software
Industry-leading data acquisition and analysis tool

GxP Compliance & Validation
Measurements verified with SpectraTest validation plate and software

Robotic Automation
Integrate with stackers and partner automation systems
Absorbance applications and assays
With more than 40 years of plate reader expertise and life science research, we’ve amassed an extensive collection of application-focused content in our Resource Hub. Our featured absorbance application notes include:
- Cell Viability, Cytotoxicity, Cell Proliferation
- -MTT Assays
- -XTT Assays
- -MTS Assays
- DNA/RNA Quantitation
- Enzyme Kinetics, Bacterial / Microbial Growth
- Endotoxin Assays
- ELISA / Immunoassays
- -Quantifying antigens, antibodies, cytokines, etc.
- Food and Beverage Applications
- Micro-volume Applications
- Protein Quantitation
- -BCA Assays
- -Bradford Assays
- -Lowry Assays
Absorbance single-mode reader product comparison
wavelength-ranges
microplate-types
reading-speed
cuvette-port
photometric-accuracy
shaking
What’s the difference between an absorbance spectrophotometer and a microplate reader?
A standard spectrophotometer measures the absorbance of one sample at a time. The sample is typically placed in a cuvette through which light is sent horizontally. An absorbance plate reader offers higher throughput and can measure the absorbance of samples in microplates (typically 96-well or even 384-well) by sending light through each well vertically.
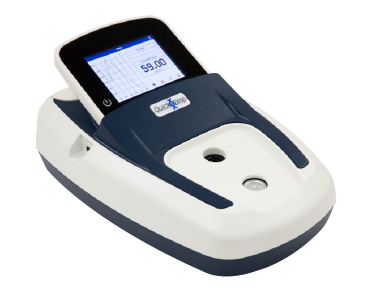
SpectraMax QuickDrop Micro-Volume Spectrophotometer
Rapid, accurate DNA, RNA, and protein quantitation in a one-touch, full-spectrum micro-volume absorbance reader.
Featured absorbance resources
APPLICATION
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA (enzyme-linked immunosorbent assay) is a method used to quantitatively detect an antigen within a sample. An antigen is a toxin or other foreign substance, for example a flu virus or environmental contaminant, that causes the vertebrate immune system to mount a defensive response.

TECHNOLOGY
Absorbance
Absorbance (A), also known as optical density (OD), is the quantity of light absorbed by a solution. Transmittance is the quantity of light that passes through a solution.

WEBINAR
Exploring absorbance-based assay applications: from virus to cannabis research
Absorbance, also known as optical density, is the quantity of light absorbed by a solution. Microplate readers with absorbance read capability are widely used in biological and chemical research, drug discovery and quality control.
Absorbance Assay eBook
Streamline absorbance assays for nucleic acid & protein quantitation
Absorbance microplate readers are widely used in basic research, drug discovery, bioassay validation, quality control, and manufacturing processes in pharmaceutical, biotech, food and beverage, and academic industries.

FOOD & BEVERAGE EBOOK
Streamline beer, wine, and food quality control and safety analyses
Gain new insights with small but mighty absorbance microplate reader applications.
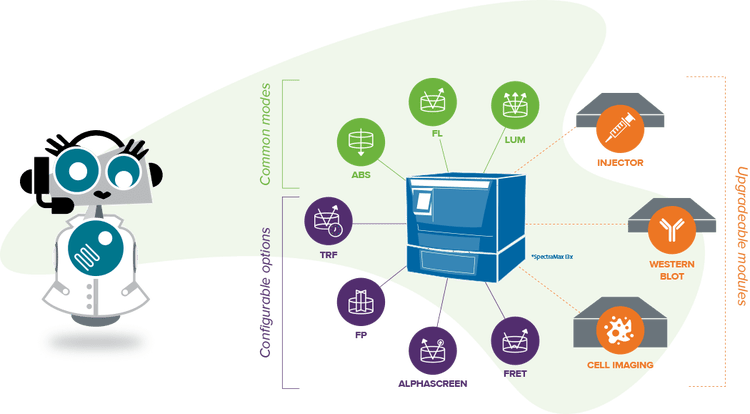
Multi-mode microplate readers with absorbance detection

Related products and services
Complete solution of tools and services, as well as a broad range of consumables and assays for all your spectrophotometer lab needs.

Software
Microplate reader control and data analysis software with preconfigured protocols and custom assay workflow.

Assay Kits
Easy-to-use, robust assay kits for life science research drug discovery and development, and bioassays.
Consumables
Assortment of high-performance labware including cartridges, modules, filters and microplates to name a few.

Services
Customize your instruments, as well as automate entire workflows to meet the specific needs of your assay, method, or protocol.
GxP compliance solutions
Ensure ongoing compliance and be audit ready with our IQ/OQ/PM validation and compliance services.

Multi-mode Plate Reader
Absorbance, fluorescence, and luminescence detection with upgradeable high-performance capabilities.
