
Absorbance
What is absorbance?
Absorbance (A), also known as optical density (OD), is the quantity of light absorbed by a solution. Transmittance is the quantity of light that passes through a solution. Absorbance and % transmittance are often used in spectrophotometry and can be expressed by the following:
Absorbance equation
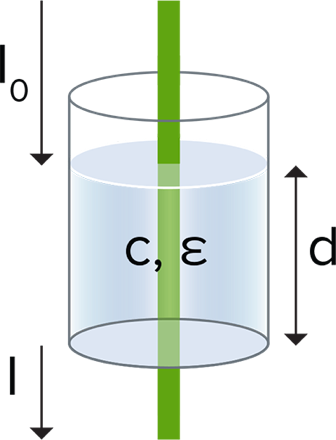
A = Log10(I0/I)
where I0 is the intensity of the incident light, and I is intensity of that light after it passed through the sample
T = I/I0 and %T = 100 (T)
The equation that allows one to calculate absorbance from % transmittance is
A = 2 - log10 (%T)
Determine concentration using the Beer-Lambert Law
The concentration of a sample can be calculated from its absorbance using the Beer–Lambert law, which is expressed as follows:
A = ε * c * p
Where ε is the molar absorptivity, or molar extinction coefficient, in L mol-1cm-1
c is the concentration of the solute in solution, in mol/L
p is the path length of the sample, in cm, for example 1 cm for a cuvette
Ultraviolet (UV) measurements in microplates became possible when Molecular Devices introduced the first UV-capable microplate reader. Since then, the microplate measurements of DNA, RNA, and proteins that this enabled have become very popular. Learn more about how absorbance is measured, and some key applications that utilize absorbance.
ON-DEMAND WEBINAR
Exploring absorbance based assay applications: from virus to cannabis research
Absorbance reading with SpectraMax® microplate readers and SoftMax® Pro Software enables optical density measurement of samples in microplate or cuvette format. The powerful analysis tools in the software generate a standard curve and calculated data. For the GMP/GLP regulated labs, the systems come equipped with our SoftMax Pro GxP compliance software.
Key highlights:
- How does absorbance detection work?
- Detect immunoglobulins (Ig) with ELISA – More ways to investigate infectious diseases and immune responses
- Streamline analysis of beer and wine measuring absorbance
- Determine total aflatoxin in cannabis using an ELISA
