Lab Automation 101: Inside Access From Our Subject Matter Expert
In life sciences, more is more. Obtaining a great body of data that is consistent and insightful to get a qualitative and quantitative overview of your model is critical. However, there are many challenges in running experiments with large datasets, including the error-prone nature of manual entry, environment control, and time consumption, to name a few. Fortunately, lab automation can do wonders for your research project efforts.
For those unfamiliar with the term, lab automation integrates new technologies to improve experimental capacity and efficiency. Concept-wise, it sounds great, but how does it translate to real life?
We sat down with Constantin Radu, global proposals manager at Molecular Devices, who opened our eyes to the endless opportunities of lab automation with examples from our recent collaborations.
Could you introduce yourself and your role at Molecular Devices?
Yes, absolutely; my name is Constantin Radu. I am the Global Proposals Manager at Molecular Devices automation and customization team and have worked with the company for more than four years.
Before Molecular Devices, I spent many years in the industry doing various screens. My career started at Memorial Sloan Kettering Cancer Center as a lab manager and automation scientist, implementing and monitoring various screening campaigns. Over the years, I have gained lots of experience running, maintaining, and using automation platforms. The experience gives me expert insights into scientific workflows and understanding the logistics of automating those workflows.

My role was to work with the assays developed by scientists and figure out how to manage them from an engineering perspective. These assays were developed in microtiter plates. We had to screen approximately 400,000 small molecules in thousands of plates. My objective was to figure out how to run these assays on large platforms.
Those types of innovative projects translated significantly with my current position at Molecular Devices. My role is to consult and understand our customers' application requirements, determine what their workflows look like, and what throughput they want to achieve today and in the future. Then, I propose a working solution for their specific needs, including labware, lab robotics, and software solutions.
Why would scientists want to automate their workflow?
There are many reasons to automate your workflow, but the top six reasons include:
- Consistency in data acquisition
- Flexibility
- Reducing human errors
- Improving lab safety
- Increasing walk-away time
- Enhancing throughput
Consistency in data acquisition is one thing. Let’s say you run a time course on your plates for 48 to 96 hours, and you collect data every 6 hours. Waiting in the lab all night isn’t feasible. Instead, an automated system provides a solution where the plates come in and out of an automated incubator for imaging or measurement at scheduled time points.
A robotic arm in the automated system never gets tired, but humans do. We can sometimes forget to read up or image a plate at a certain time. In the end, it’s inconsistent for your data acquisition.
Another benefit to automating workflows is the increased walk-away time. When experiments are running on the automated platform, scientists can utilize that time more productively to write grants, work on scientific papers, or focus on other research-related aspects rather than constantly being in front of the bench.
These improvements eventually lead to more presentable results in shorter time frames—enhancing throughput—and can accelerate publications, which are, after all, the currency of a scientist and the key to collaborations and increased funding. I witnessed firsthand how lab automation contributed to the networking and funding flow at Memorial Sloan Kettering Cancer Center.
How does lab automation add value to various types of assays?
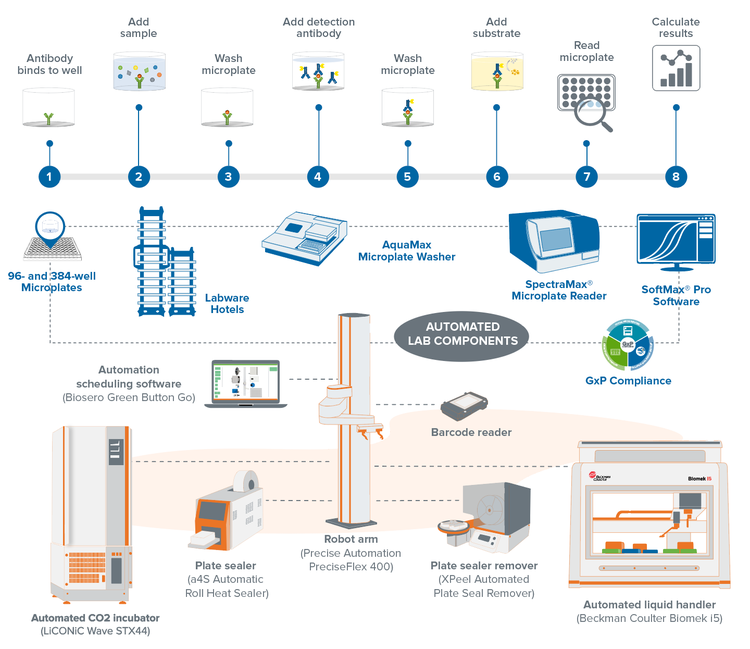
An ELISA, for example, can benefit tremendously from lab automation and become part of a high-throughput plate-based assay workflows. During the screening of live cells with compound libraries, which requires an incubator, environment control becomes a major challenge because the incubator is highly sensitive to changes. The temperature needs to be at 37°C with 5% CO2. If these values deviate, it could ruin the experiment. Keep in mind that the assay procedure itself is labor-intensive.
After customers purchase the basic ELISA system, we set up an automation-ready ELISA workflow for them and follow a process that may look similar to this:
- The workflow started with automated liquid handling for dispensing reagents and samples. It also involved an automated heat sealer that retained the temperature and prevented evaporation during incubation.
- The plates were transferred to seal-peelers, followed by an automated microplate washer, performing simultaneous washing of 96- and 384- well plates.
- The workflow automatically generated absorbance readouts in microplate readers used to calculate the target antigen concentration of the samples.
The main takeaway is that the automated workflow brought easiness and peace of mind to what would have been a very intricate procedure every step of the way.
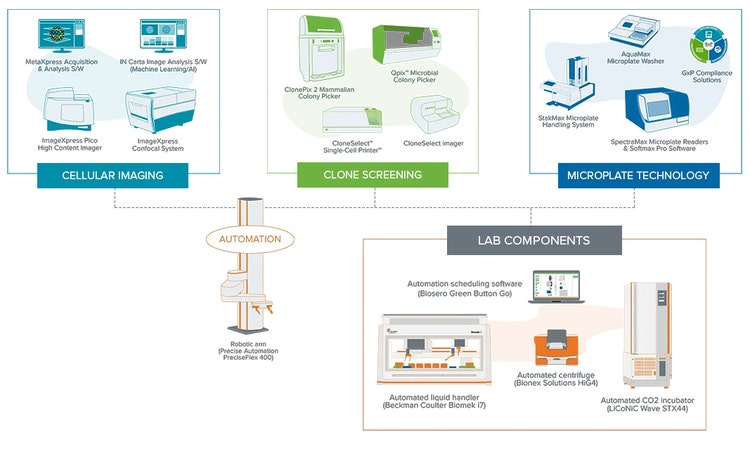
I believe cell line development is another perfect example where we can benefit from automation because you characterize and analyze up to millions of cells. We developed automated clone screening workflows, which allowed seamless cell line development from single cell-derived clones while ensuring all clones produced high target protein levels. Again, the integrated solution involved coordination between single-cell printing, imager, microplate reader, and the automated incubator. You can learn more about our high-throughput clone screening solutions on our website, but similar to the ELISA example, you end up saving a lot of time and resources.
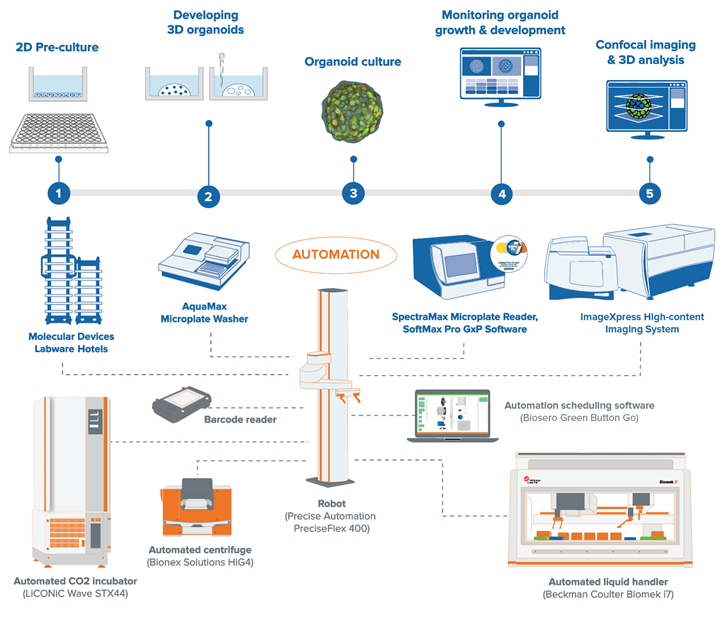
Could you describe your involvement in Molecular Devices’ Organoid Innovation Center?
My involvement started with a collaboration between Molecular Devices and Cincinnati Children’s Center for Stem Cell and Organoid Medicine (CuSTOM). Basically, we approached them to identify their needs and goals. At the time, their research group was a pioneer in organoid research. They wanted to automate their workflow for 3D cell culturing and organoid screening to improve their research outcomes in personalized medicine.
The instruments they used – incubator, ambient, and storage tools – were nothing new. However, our automated workflow methodologies helped them optimize the communication between these instruments.
Afterward, we realized a common trend among our new customers: a desire to work with biologically relevant samples like organoids. The challenge was that they didn’t know how to get reproducible results. Plus, there was no standardized procedure to grow, image, and analyze these complex samples.
With the Organoid Innovation Center (OIC) establishment, we started to assist our customers by demonstrating 3D automated workflows for their specific assays in real-time. CuSTOM was able to witness every step, from cell culturing to differentiation and screening.
Before the screening, we also ran stability tests on their reagents. Someone might have needed to come in at certain intervals to prepare new buffers, so we tested how long a buffer would remain stable. We also trained our customers on how to use their designated workflows and run stability tests.
https://share.vidyard.com/watch/WrM2vds8jA6Ayc2joqUDnR
To summarize, the OIC became a platform for our customers to bring their research goals and desired methodologies to life.
What makes Molecular Devices different from competitors in customizing lab automated solutions?
At Molecular Devices, we take the time to drill down on our customer’s workflows and understand the application and end goal. We demonstrate a model simulation of their ideal workflow. What’s fascinating about the lab automation at the OIC is its ability to mimic the manual steps a lab member would perform for organoid research. This feature makes that demonstration more convincing. We also provide written reports of our instrument and workflow tests afterward. Then, the customers feel assured that this workflow is a good fit.
Our service engineers provide worldwide customer support 24/7. In addition, our service team is the best-in-class and offers continual support post-installation.
Getting timely global support from a US-based company can be challenging. For example, I used to work in a research lab in South Korea, but the engineering support would come from Germany, and the parts from Singapore—it would take several months to get our imagers repaired. At Molecular Devices, we work diligently to eliminate the delays arising from different time zones.
Another standout example of our automated solutions is the flexibility with integrated lab automation systems. We use Molecular Devices instruments and other third-party instruments that complement the researcher’s scientific needs.
What is the future of lab automation?
Automation is not new; it has been used since the mid-1990s for drug screening. The difference back then was that there were multiple vendors developing imagers and plates of different sizes, so not every lab automation was compatible with every instrument.
Lab automation has enormous potential and room for discoveries. The next goal is to enhance user experience through sophisticated mobile robots that could assist the scientists as they run the workflows or work side-by-side with researchers.
I know from experience that to take care of your cells, you sometimes need to go to the lab on Saturdays and Sundays. With COVID-19 restrictions, work hours must be more flexible for researchers. Lab automation will come in handy, particularly in the new normal, so you will not need to be physically in the lab daily and around the clock. Automation will allow room for productivity, thanks to the increase in walkaway time, allowing you to multitask since automated robotics will do most of the heavy lifting.
Explore automation-ready systems
Regardless of the scientific question or application at hand, an automated workflow can bring answers faster. By integrating leading technology from across the industry into a flexible, customizable work cell, researchers can greatly optimize their time in the lab and free up resources for other critical tasks, speeding discovery.
Explore lab automation-ready workflows in an end-to-end, high-throughput screening solution for the following:
- High-content cellular screening
- High-throughput clone screening
- Protein and cell biology
https://main--moleculardevices--hlxsites.hlx.page/service-support/lab-automation-solutions
From incubators, liquid handlers, and robotics to customized software and hardware—and with over 35 years of experience in the life science industry—you can count on us to deliver quality products and provide worldwide support.
Our Global Professional Services teams strive to earn customer loyalty through the enablement of their success with empowered people and innovative solutions. From our Ph.D. level Technical Support to our expert-trained and certified Field Service Engineers, we've built a world-class team to support our customer's research pursuits, so they can help answer life science's most important questions.