Cardiotoxicity
Predictive in vitro assays for high throughput assessment of cardiac toxicity and drug safety
What is cardiotoxicity?
Cardiotoxicity or cardiac toxicity is a term used to define chemicals that are toxic to the heart, causing muscle damage or heart electrophysiology dysfunction.
The assessment of cardiotoxicity is important in the early stages of drug discovery to eliminate potentially toxic compounds from further development. This is critical to reduce the inefficiencies and high costs associated with compounds that fail during cardiac safety assessment.
Cardioactive compounds are used in clinical treatment of heart failure, arrhythmia, or other cardiac diseases. Cardiac toxicity can cause arrhythmias or heart failure. Therefore, there is a growing need for highly predictive in vitro cardiotoxicity assays that use biologically relevant 3D cell-based models and are suitable for high-throughput screening.
Evaluate cardiotoxic compound effects using human iPSC-derived cardiomyocytes
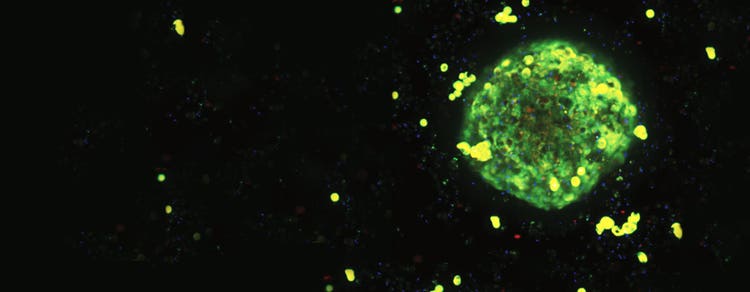
What are cardiomyocytes?
Cardiomyocytes are the cells that make up the cardiac muscles and are responsible for the heart's contractile function. Specialized cardiomyocytes like human induced pluripotent stem cell (iPSC)-derived cardiomyocytes are especially attractive cell models because they express GPCRs and ion channels that provide gene expression profiles as well as phenotypic characteristics while demonstrating spontaneous mechanical and electrical activity similar to native cardiac cells. iPSC-derived cardiomyocytes form a synchronously beating monolayer that can be used to reliably reproduce drug-associated, cardio-physiologic phenotypes using a fast, kinetic fluorescence assay that monitors changes in intracellular calcium oscillations.

In collaboration with Eurofins, we discuss the assessment of potential cardiotoxicity of compounds with CiPA initiative, the new cardiac safety testing paradigm that includes in vitro assays using hiPSC-CM.
The CiPA Initiative – Comprehensive in vitro Proarrhythmia Assay
Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) can be used to identify and evaluate the effects of potentially cardiotoxic compounds. The US FDA has sponsored an initiative to evaluate compounds in this category called the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative. There are 28 compounds that have various effects on cardiomyocytes that can be toxic. The purpose of this initiative is to determine assays that are predictive earlier in the drug discovery process to help prevent failure in either late-stage development or even after release. Several compounds on the CiPA list have been pulled from the market due to cardiac toxicity. It is possible to identify toxicity in these compounds using both measurement of calcium flux, and high content imaging. The development of highly predictive in vitro assays suitable for high-throughput screening is critical to improving the inefficiencies and high costs associated with cardiac safety compound failure.
High-throughput kinetic screening solution for toxicology and lead compound identification
The FLIPR® Penta High-Throughput Cellular Screening System is a flexible and reliable real-time kinetic high-throughput cellular assay screening system for identifying early leads against GPCR and ion channel receptors. It is designed to collect signals from all wells at the same time. The system can also monitor changes in intracellular Ca2+ flux (calcium oscillations) associated with cardiomyocyte contractions using the EarlyTox Cardiotoxicity Kit. The assays use iPSC-derived cardiomyocytes loaded with a calcium-sensitive dye and allow you to monitor the compound impact on oscillating calcium within the cells as they beat.
ScreenWorks® Peak Pro Software offers the ability to analyze multi-peak calcium oscillation responses of cells—in particular, cardiomyocyte beat rate as well as temporal characteristics of peaks including rise, decrease, and amplitude. These features are important to better understand in vitro cardiomyocyte function and the impact of toxic compounds that induce cardiac abnormalities such as hERG blocking potassium channels. As a result, you can fail or further monitor specific compounds sooner, and better prioritize the most promising, safe, leads to take forward into preclinical studies.
Spontaneous calcium oscillation acquired with FLIPR Calcium Assay Kit and the FLIPR system from iPSC cardiomyocytes or 3D cardiac spheroids can be paired with cytotoxicity and mitochondrial integrity data from the ImageXpress confocal system for comprehensive phenotypic analysis and bioactivity profiling of both pharmaceutical and environmental compounds.
Applications for characterizing cardiotoxic compounds using stem cell-derived cardiomyocytes
Traditional methods for characterizing cardiotoxic compounds are labor-intensive and slow. Manual patch clamping and automated electrophysiology are limited to the analysis of single channels on individual cells and have high costs and low throughput. Other, higher throughput methods require the exporting of data into additional, often complex, software for analysis or time-consuming manual analysis of the data.
Our application assets below demonstrate how ScreenWorks Peak Pro Software running on the FLIPR system with EarlyTox Cardiotoxicity Kit can quickly and easily characterize cardiotoxic compounds using stem cell-derived cardiomyocytes. In addition, the FLIPR system can be used to analyze neurotoxic effects upon Neuronal spheroids in much the same manner as iPSC-derived cardiomyocytes.