
Accelerated Production of Monoclonal Antibodies for the Treatment of SARS-CoV-2
With solutions from Molecular Devices, scientists can fast-track the FDA approval process and accelerate monoclonal antibody discovery
The timeline to identify and develop clinically effective neutralizing antibodies against viral particles from bench to patient is typically 18 to 24 months. The worldwide race to identify effective neutralizing antibodies against the spike protein of SARS-CoV-2 virus became a life and death matter as the global death toll climbed in the first year of the COVID-19 pandemic.
Using a combination of CloneSelect instruments from Molecular Devices, with the CloneSelect™ Single-Cell Printer™ and CloneSelect Imager, the timeline for preclinical development of effective neutralizing antibodies can be significantly shortened. In fact, the speed at which the pharmaceutical world was able to create and deploy SARS-CoV-2 monoclonal antibodies speaks to the power and promise of cell line development automation.
In this article, we demonstrate the steps to develop monoclonal antibodies with Molecular Devices instruments from clonal selection to cell growth tracking to image-based assurance of monoclonality. An image-based monoclonality report is then generated to be included in global licensing applications such as the Biologics License Application (BLA) to the US Food and Drug Administration (FDA).
Monoclonal Antibodies as an Alternative to Convalescent Plasma Therapy
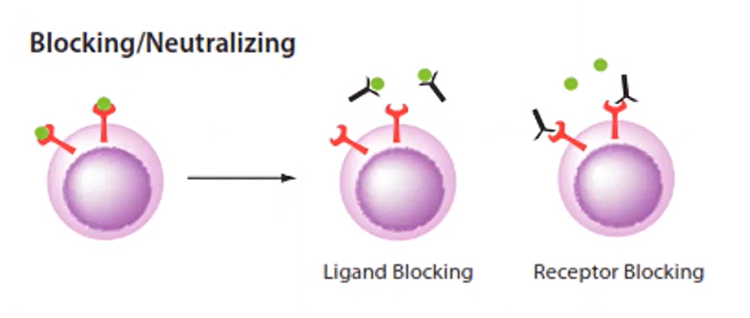
More recently, monoclonal antibodies, laboratory-made antibodies cloned from white blood cells, are available for use to treat the SARS-CoV-2 virus. The word monoclonal describes a cell line that originates from a single progenitor (single cell) and documenting evidence of clonality is required for regulatory filing. Preliminary results showed that these antibodies provide immunological support for at least 5-7 months [5]. Given the therapeutic potential, fast and efficient monoclonal antibody production has become increasingly crucial.
There is one problem with monoclonal antibodies: the time from discovery to proof-of-concept trials. The timeline from the identification of the antibody through to the IND Phase-I trial can take up to 10-12 months [6]. However, with fast and efficient transfection, selection, and clone screening, there are ways to accelerate the process and reduce the production timeline by half.

Figure 2 - The time from discovery to proof-of-concept trials could be reduced to 5–6 months from a traditional timeline of 10–12 months.
Accelerated Monoclonal Antibody Development Workflow
As regulations for cell line development become increasingly more stringent, researchers will be required to perform single-cell cloning and provide evidence that a cell line is derived from a single cell—proof of clonality. Traditional cloning methods (e.g., limiting dilution and FACS) use statistical analysis to determine a confidence level for monoclonality. However, the documentation of monoclonality has driven the need for more robust technologies and methodologies in bioprocessing. Many researchers now routinely use imaging systems, such as the CloneSelect Imager, to verify monoclonality and monitor cell growth in cell culture media.
An ideal production workflow that generates acceptable monoclonality assurance consists of the following steps.

Figure 3 - Monoclonal antibody development workflow
Challenge 1: Single-Cell Isolation
The key to robust monoclonal antibody production is to isolate individual high-performing cells. However, there are various challenges of single-cell isolation, such as obtaining a sufficient number of target cells and maintaining cell viability.
Limiting dilution (LD) is a traditional isolation method, where the parent cell culture undergoes a series of dilutions until there is one cell per one plate in a well based on probability calculations. The main drawback comes from its low efficiency of isolation, meaning that the number of cells per plate fluctuates between zero and multiple cells [7].
Flow cytometry (FC) is a more successful isolation method that implements fluorescence-activated cell sorting, but it compromises cell viability, due to high pressure, exposure to electric charge, and frequent high-speed collisions. The unviable conditions lead to what is known as sorter-induced cellular stress (SICS) [8]. So, if you are working with sensitive cell types, the cell line obtained from flow cytometry may not be suitable for further research and set your timeline back by months.
Microfluidics, the process of isolating single cells in microfluidic chips, has been emerging as a more efficient alternative to conventional methods. One factor that makes microfluidics stand out is the massive reduction in input volumes, as you are loading your samples into microliter chips. Since the microchips have lower sorting pressure, the SICS risk is also reduced. Finally, microfluidics eliminates the risk of sample-to-sample contamination [9].
We recently compared the single-cell efficiency of these three methods. For the demonstration of microfluidics-based isolation, we used our CloneSelect Single-Cell Printer that combines the technology with high-resolution imaging to isolate individual cells while recording image-based evidence of monoclonality. As you can see in the bar chart below, the CloneSelect Single-Cell Printer outperformed LD and FC by 8-fold and 10-20%, respectively.

Figure 4 - Single Cell Efficiency: the f-sight has superior plating efficiency to LD and FC.
Challenge 2: Colonial Outgrowth Efficiency
The next question is: How does single-cell efficiency translate when you are trying to generate monoclonal colonies?
For the colonial outgrowth comparison of microfluidics to LD and FC, we used the CloneSelect Imager, which allowed us to scan division from a single cell for 90 seconds. The CloneSelect Imager provided both chart statistics and visual thumbnails of cell growth as evidence. Since the CSI enables subsequent scanning of the plate for 14 days, you can easily trace back from the final image to day zero to prove that the colony indeed formed from a single cell. In addition, you can export your colony images as a PDF or Word report for image-based monoclonality assurance.
So, how does this contribute to reducing the timeline? Instead of two-round isolation for providing probability-based and image-based assurance, you could use a single-cell printer and imager synergistically for one round of cloning. This method was proven to confer monoclonality assurance with over 99.99% confidence [10].
The outgrowth efficiency results are represented below for both freestyle and recombinant CHO cells.

Figure 5 - Colonial outgrowth efficiency: the f.sight shows over five times improvement of clonal outgrowth versus traditional limiting dilution.
Similar to single-cell efficiency, LD performed poorly for both cell lines. Interestingly, FC displayed an almost equal efficiency to CloneSelect Single-Cell Printer in recombinant cells but 15-fold worse in free-style cells. This clearly indicates that the CloneSelect Single-Cell Printer™, coupled with the CloneSelect® Imager technology, is more consistent in terms of colonal outgrowth than FC.
Challenge 3: Large Scale Monoclonality and Secretion
Isolation of candidate mammalian cell clones can be strenuous and time-consuming, as you need to screen hundreds of thousands of clones not only for monoclonality but also for sufficient secretion of antibodies.
Molecular Devices has developed the ClonePix® System for an end-to-end automated cell line development workflow with proof of monoclonality. The ClonePix System is equipped to screen and quantify neutralizing antibodies secreted from a large number of CHO clones in situ. The software uses a fluorescent detection probe to quantify secretion and automatically picks the clones with the highest yield.
As illustrated below, the ClonePix® outperforms LD in several aspects. Not only can it screen a substantially greater number of colonies using fewer plates, but it also picks colonies that have higher yields.

Figure 6 - ClonePix: in situ allows cumulative date rather than single-time point to determine productivity.
What about timeline reduction? The original version of the ClonePix2 Mammalian Colony Picker workflow requires two rounds of screening, as suggested by FDA, in concert with the CloneSelect Single-Cell Printer and Imager combination.
Explore lab automation-ready workflows
Let’s make your ideas a reality! Our customization and automation team has successfully customized the ClonePix 2 Mammalian Colony Picker for customers by request—including software and hardware. For example, by updating the system with additional monoclonal assurance capabilities, a single instrument can serve all monoclonal antibody production workflow needs from antibody screening to titer with one round of clone screening.

With Molecular Devices automated clone screening workflows, you can ease the burden on your lab by significantly reducing hands-on-time while creating a central repository for data pulled from multiple processes. Our automated solutions unify all of your laboratory devices to increase your throughput and efficiency while reducing human interaction.
References
- Mair-Jenkins, John, et al. "The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis." The Journal of infectious diseases 211.1 (2015): 80-90.Mair-Jenkins, John, et al. "The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis." The Journal of infectious diseases 211.1 (2015): 80-90.
- Ko, Jae-Hoon, et al. "Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience." Antivir ther 23.7 (2018): 617-622.Ko, Jae-Hoon, et al. "Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience." Antivir ther 23.7 (2018): 617-622.
- Shen, Chenguang, et al. "Treatment of 5 critically ill patients with COVID-19 with convalescent plasma." Jama 323.16 (2020): 1582-1589.Shen, Chenguang, et al. "Treatment of 5 critically ill patients with COVID-19 with convalescent plasma." Jama 323.16 (2020): 1582-1589.
- Gontu, Abhinay, et al. "Limited window for donation of convalescent plasma with high live-virus neutralizing antibody titers for COVID-19 immunotherapy." Communications biology 4.1 (2021): 1-9.Gontu, Abhinay, et al. "Limited window for donation of convalescent plasma with high live-virus neutralizing antibody titers for COVID-19 immunotherapy." Communications biology 4.1 (2021): 1-9.
- Ripperger, Tyler J., et al. "Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity." Immunity 53.5 (2020): 925-933.Ripperger, Tyler J., et al. "Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity." Immunity 53.5 (2020): 925-933.
- Kelley, Brian. "Developing therapeutic monoclonal antibodies at pandemic pace." Nature Biotechnology 38.5 (2020): 540-545.Kelley, Brian. "Developing therapeutic monoclonal antibodies at pandemic pace." Nature Biotechnology 38.5 (2020): 540-545.
- Heisler, E., and H. W. Vohr. "3D Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems. The Encyclopedic Reference of Immunotoxicology." (2005).Heisler, E., and H. W. Vohr. "3D Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems. The Encyclopedic Reference of Immunotoxicology." (2005).
- Hu, Ping, et al. "Single cell isolation and analysis." Frontiers in cell and developmental biology 4 (2016): 116.Hu, Ping, et al. "Single cell isolation and analysis." Frontiers in cell and developmental biology 4 (2016): 116.
- Gross, Andre, et al. "Technologies for single-cell isolation." International journal of molecular sciences 16.8 (2015): 16897-16919.Gross, Andre, et al. "Technologies for single-cell isolation." International journal of molecular sciences 16.8 (2015): 16897-16919.
- Yim, Mandy, and David Shaw. "Achieving greater efficiency and higher confidence in single‐cell cloning by combining cell printing and plate imaging technologies." Biotechnology progress 34.6 (2018): 1454-1459.Yim, Mandy, and David Shaw. "Achieving greater efficiency and higher confidence in single‐cell cloning by combining cell printing and plate imaging technologies." Biotechnology progress 34.6 (2018): 1454-1459.