
Monoclonal Antibodies (mAbs)
Monoclonal antibody (mAb) discovery
Monoclonal antibodies (mAbs) originate from one unique parent cell, thus binding only to a single epitope. Monoclonal antibody discovery typically refers to the screening and identification of specific antibodies that target a specific epitope for the diagnosis and treatment of diseases, like the coronavirus for COVID-19.
There are several approaches to generating therapeutic mAbs. Two of the most common production methods are hybridomas and phage display.
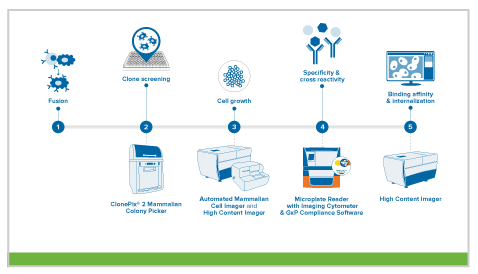
mAb production via hybridomas
In this approach, scientists fuse immortalized myeloma cells with splenocytes obtained from animals previously exposed to the antigen of interest. The resulting fused cell is called a hybridoma. The advantage of a hybridoma is that it now possesses the ability to produce mAbs while dividing indefinitely, whereas splenocytes have a finite lifespan. Researchers then screen thousands of hybridomas to identify the optimal ones based on antigen specificity and immunoglobulin class. These candidates are subsequently analyzed, characterized, and scaled up for downstream processes.
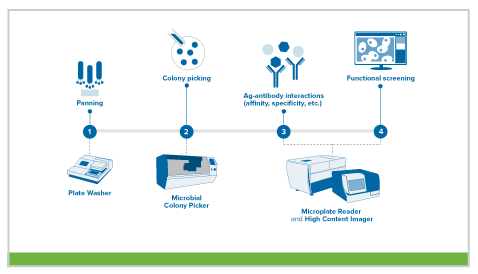
mAb production via phage display
In this method, the variable heavy and light genes of an antibody are introduced into a bacteriophage coat protein gene, resulting in phages that display the antibody on their surface. Using this strategy, scientists can generate an antibody library displaying millions of different antibodies. These displaying phages are then screened against the antigen of interest, and those that are highly specific are identified, isolated, and further characterized for downstream work.
How are monoclonal antibodies produced?
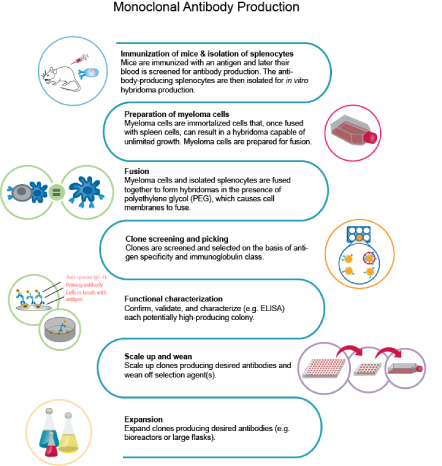
The traditional monoclonal antibody (mAb) production process usually starts with generation of mAb-producing cells (i.e. hybridomas) by fusing myeloma cells with desired antibody-producing splenocytes (e.g. B cells). These B cells are typically sourced from animals, usually mice. After cell fusion, large numbers of clones are screened and selected on the basis of antigen specificity and immunoglobulin class. Once candidate hybridoma cell lines are identified, each "hit" is confirmed, validated, and characterized using a variety of downstream functional assays. Upon completion, the clones are scaled-up where additional downstream bioprocesses occur.
Additional resources for antibody discovery
Accelerate your monoclonal antibody discovery with our product portfolio specifically designed to shorten cell line development time and deliver stable clones with higher affinities and expression levels.