
Monoclonality
Document monoclonality – a regulatory metric for therapeutic cell lines
What is monoclonality?
Monoclonality is term that describes a cell line that originates from a single progenitor (single cell) - and is therefore monoclonal. Cell line development and assurance of monoclonality are critical steps in the process of generating biopharmaceutical molecules, such as monoclonal antibodies. A cell line can be established following the isolation of a single viable cell robustly expressing the protein of interest. A key milestone in this process is documenting evidence of clonality to ensure the genetic reproducibility of the cell line.
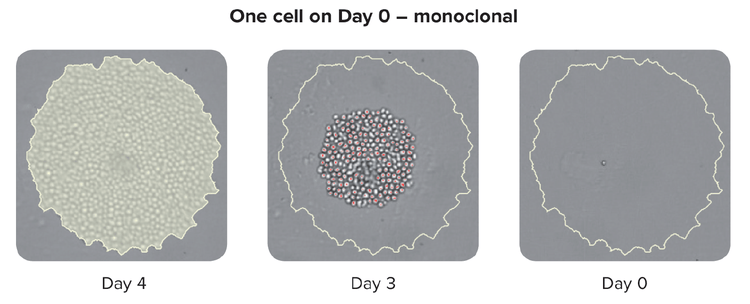
Documentation of monoclonality is a regulatory metric for therapeutic cell lines and is typically image-based, whereby an image of a single cell is recorded and included in regulatory filings.
Why is monoclonality important?
As regulations for cell line development become increasingly more stringent, researchers will be required to perform single-cell cloning and provide evidence that a cell line is derived from a single cell—proof of clonality. Traditional cloning methods (e.g. limiting dilution and FACS) use statistical analysis to determine a confidence level for monoclonality. However, the documentation of monoclonality has driven the need for more robust technologies and methodologies in bioprocessing. Many researchers now routinely use imaging systems, such as the CloneSelect Imager, to verify monoclonality and monitor cell growth in cell culture media.
High assurance monoclonality for cell line development
The development of cell lines that express a specific protein of interest is critical to the generation of biologics, and regulatory agencies require evidence of monoclonality in order to get a biologic to the marketplace. The traditional approach involves visualization of wells using transmitted white light on day 0 to confirm the presence of a single cell. However, this approach is not without challenge, as cellular debris and well artifacts can be easily mistaken for cells. Consequently, cell line developers typically evaluate cells at the colony state and trace back the origins of the colony to confirm monoclonality.
The workflow highlights the process of CloneSelect Imager System to verify monoclonality based on objective image analysis. Coupled with the DispenCell Single-Cell Dispenser for single-cell sorting improves the workflow with both an increase in clonal outgrowth and additional image-based assurance of monoclonality.
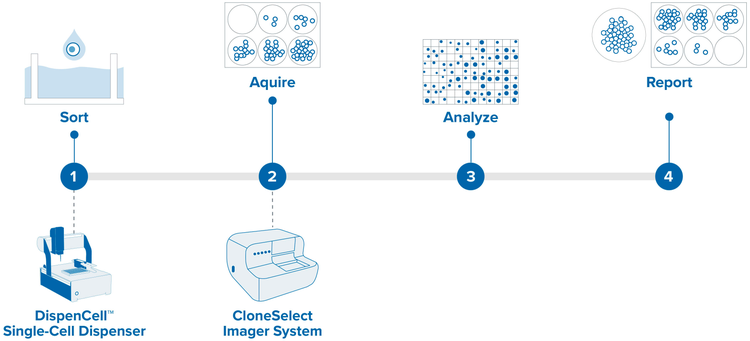
- Sort single cells into microplate wells – Single, viable cells must be isolated and cloned in order to ensure that the cell population is genetically identical, significantly reducing the heterogeneity of expression.
- Scan plate WL at Day 0 and subsequent days – Image plate at Day 0 and subsequent days. Documentation of monoclonality (a regulatory metric for therapeutic cell lines) is typically image-based, whereby an image of a single cell is recorded and included in regulatory filings.
- Review plate scan and assess overall outgrowth (WL) – Review and analyze images tracking cell growth rates, confluence measurements, and colony formation.
- Confirm monoclonality and export well data for regulatory submission – Confirm monoclonality and export well data in detailed report for regulatory submission.
Improve clonal outgrowth with single-cell dispensing
DispenCell’s single-cell dispensing unit is fitted with a sensing tip that detects the passage of the cells. As each cell advances, a unique electrical signal is triggered. This unique electrical trace is immediately recorded, allowing the user to check for proof of clonality immediately after the cells are dispensed.
The technology is impedance-based, thus extremely gentle with cells, preserving their viability and integrity for optimal outgrowth. The DispenCell uses a sterile disposable sensing tip (DispenTip) to sense and record the passage of every cell flowing through the DispenTip aperture. As a single cell passes through the aperture to flow into the well, it leaves an electrical signature that appears as a unique peak, whereas multiple peaks result from doublets or multiple cells.
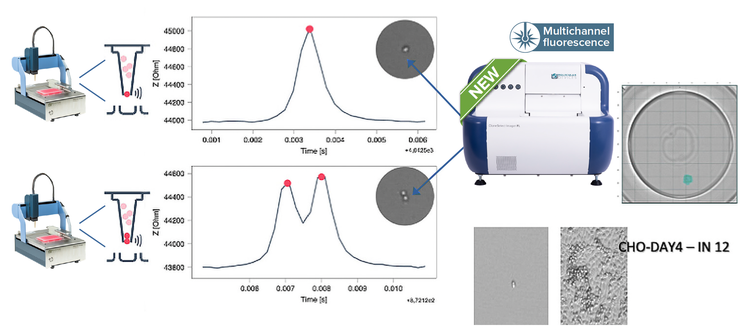
A pictorial representation of the peak information produced by the dispenser of two wells beside their images from CSI-FL. One with a single peak and cell (top) and one with a double peak and two cells (bottom). The single cell developed into a colony and its monoclonality report was generated using the monoclonality report feature on CSI-FL
What is monoclonal and what is not
By using the CloneSelect Imager software to zoom in on individual, immobilized colonies, it is possible to track growth and determine whether a colony is monoclonal by reviewing the colony images at different time points. In figure below, the images of two colonies are shown. By observing growth of the two colonies and tracking the images back to Day 0, it can be determined whether or not the colony originated from one or more cells. The addition of the beads (shown in the yellow circles) to the semi-solid media serves as a location reference to confirm that the same colony is being imaged each time.
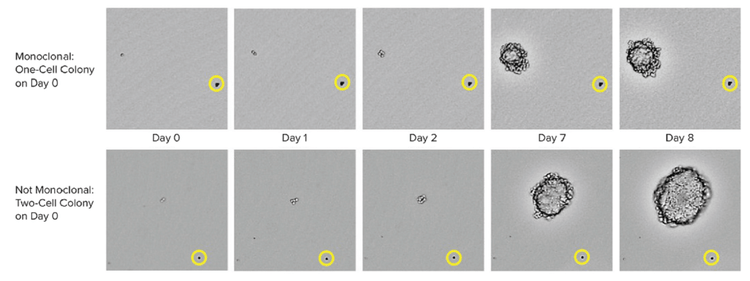
CHO-s cell growth in CloneMedia CHO Growth A semi-solid media CloneSelect imager was used to capture images from the 6-well plates at multiple time points. On day 0, it is clearly observed on the top row that one cell is present while on the bottom row, two cells are observed. The yellow circle show the position of a bead that serves as a location reference to confirm that the same colony is imaged over time.
Providing image evidence of monoclonality (report)
Providing image evidence of monoclonality in the cell line development process is not as simple as exporting an image of a single cell. For example, high-resolution images of the entire well should also be inspected to ensure the absence of a second cell.
With a few simple clicks, the Monoclonality Report feature on the CloneSelect™ Imager (CSI) objectively organizes the supporting image evidence needed to establish clonality into an easily shareable report, saving researchers hours typically required to do the same process manually.

Methods for monoclonality assurance
Learn more about the different methods to verify and document monoclonality.