Stem Cell Science and Regenerative Medicine – Technology and Methods Presented at ISSCR 2022
It was another great year at ISSCR 2022! Leaders from across the globe came together to discuss new technologies, share insights, and explore the newest breakthroughs in stem cell science and regenerative medicine. In addition, attendees learned about automated and quantifiable solutions for the complexities that come with 3D cell model assays.
In case you missed the show or would like additional details about the technology and methods presented—we've got you covered!
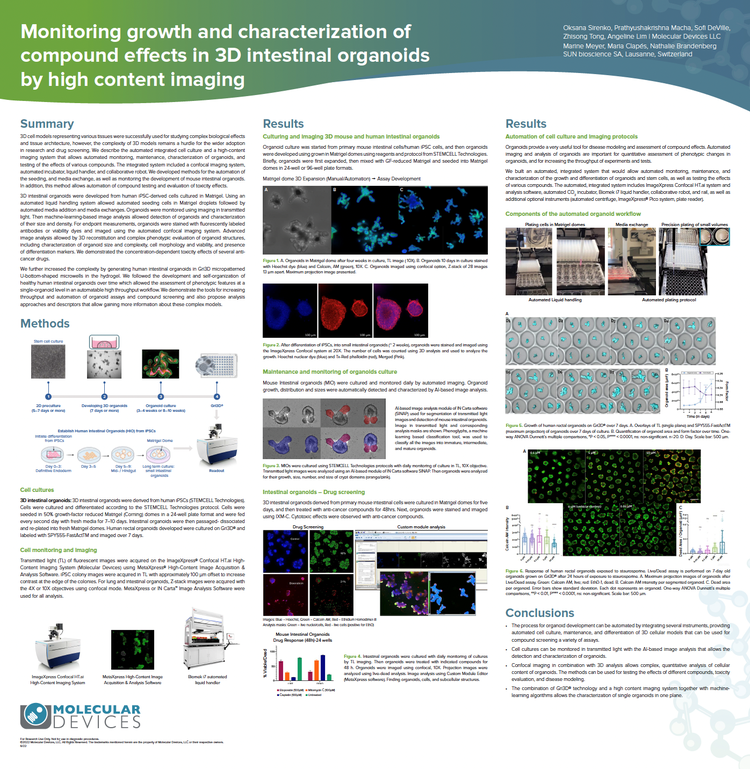
Monitoring growth and characterization of compound effects in 3D intestinal organoids with high-content imaging
We developed methods for the automation of the seeding cells in Matrigel and media exchange, as well as monitoring the development of mouse intestinal organoids using imaging in transmitted light. Machine-learning-based image analysis allowed detection of organoids and characterization of their size and density. Advanced image analysis allowed 3D reconstitution and complex phenotypic evaluation of organoid structures, including characterization of organoid size and complexity, cell morphology and viability, and presence of differentiation markers. We demonstrate the concentration-dependent toxicity effects of several anti-cancer drugs.
We further increased the complexity by generating human intestinal organoids in Gri3D® micropatterned U-bottom-shaped microwells in the hydrogel. We demonstrate the tools for increasing throughput and automation of organoid assays and compound screening and propose analysis approaches and descriptors that deliver more information about these complex models.
Structural Organization and Functional Analysis of Compound Response in 3D Human iPSC-derived Cardiac Tri-Culture Microtissues
In this study, we used a tri-culture model created by mixing iPSC-derived cardiac cells with primary adult fibroblasts and iPSC-derived endothelial cells. We used a Biomek i7 liquid handling system for cell plating and subsequent media exchange. We investigated the functional activity of microtissues by recording calcium oscillations using a fast kinetic fluorescence recording instrument - the FLIPR® Penta High-Throughput Cellular Screening System - while waveform analysis was performed using the ScreenWorks® Peak Pro 2™ software module. Additionally, we used high-content imaging to characterize the structure and morphology of our 3D microtissues with the ImageXpress® Micro Confocal High-Content Imaging System. The 3D structure of microtissues was reconstructed and analyzed using MetaXpress® High-Content Image Acquisition and Analysis Software.
The data presented here highlights the utility and biological relevance of using iPSC-derived cell types in 3D micro-tissues as a promising model for measuring compound effects on human cardiac tissues in a high-throughput format.
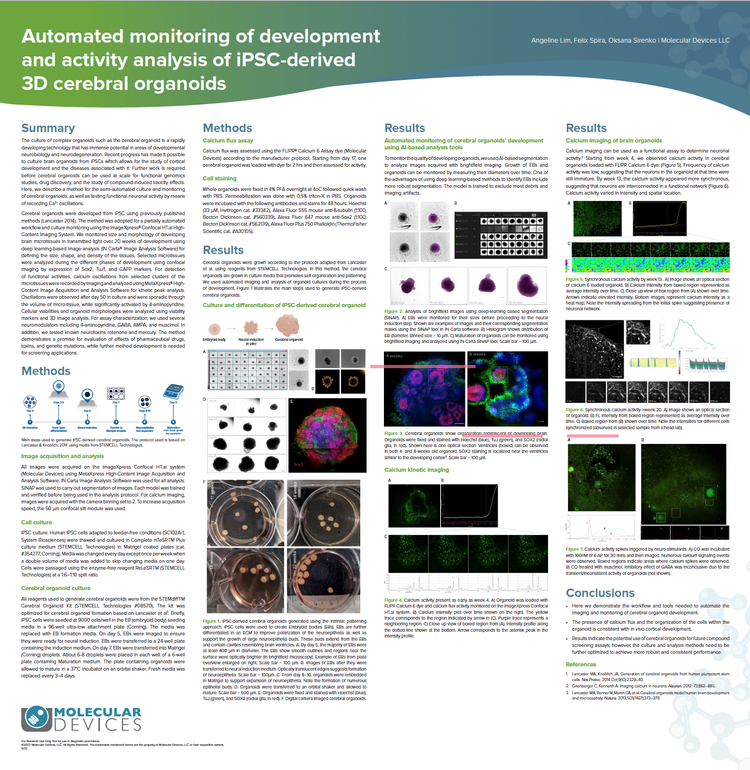
Automated monitoring of development and activity analysis of iPSC-derived 3D cerebral organoids
Cerebral organoids were developed from iPSC using previously published methods (Lancaster 2014). The method was adopted for a partially automated workflow and culture monitoring using the ImageXpress Confocal HT.ai High-Content Imaging System. We monitored size and morphology of developing brain microtissues in transmitted light over 20 weeks of development using deep learning-based image analysis (IN Carta® Image Analysis Software) for defining the size, shape, and density of the tissues. For detection of functional activities, calcium oscillations from selected clusters of the microtissues were recorded by imaging and analyzed using MetaXpress High-Content Image Acquisition and Analysis Software for kinetic peak analysis.
The method demonstrates a promise for the evaluation of effects of pharmaceutical drugs, toxins, and genetic mutations, while further method development is needed for screening applications.
Take a peek into where our research happens!
Tour our Organoid Innovation Center where we showcase cutting-edge instruments that work harmoniously together for autonomous, long-term, live cell 2D and 3D cell culture growth and monitoring with intelligent label-free imaging. This integrated workflow provides quality control alerts and readiness, 3D organoid screening, and deep-learning image analysis that reveals hidden patterns other technologies miss.