Application Note
Wells to Westerns: Investigating the cellular heat shock response
- Monitor cell proliferation without dyes
- Investigate mechanisms of cell death with fluorescent cell imaging
- Image cells, run cell-based assays, and detect western blots, all on one platform
Introduction
Investigating a cellular response can involve multiple approaches to gathering information—imaging, cell-based viability and proliferation assays, western blots to look at changes in protein expression, and more. Often multiple instrument platforms are needed to glean the necessary results, and several software packages may need to be learned in the process.
In this application note we show how data for several related cellular parameters was collected using a single instrument, the SpectraMax® i3 Multi-Mode Detection Platform. Heat shock was used as a model system to show how different detection modes, including imaging and Western blot scanning, can be used to gain insights into a multi-faceted cellular response.
Exposure of cells to higher than normal temperatures is known to activate the apoptosis pathway, as well as increase expression of the heat shock protein HSP70. CHO-K1 cells under two different growth conditions, healthy or stressed, were exposed to heat shock then assayed for proliferation, viability, and apoptosis using the imaging capability of the SpectraMax i3 system. Expression of HSP70, which is upregulated in response to heat shock, was analyzed using the ScanLater™ Western Blot Detection System.
Materials
- CHO-K1 cells (ATCC P/N CCL-61)
- Cell culture medium (Ham’s F12 with 10% fetal bovine serum and 1% penicillin/streptomycin)
- Black-wall, clear-bottom tissue culture microplates (Corning P/N 3603)
- SpectraMax i3 Microplate Reader
- SpectraMax MiniMax™ 300 Cytometer
- EarlyTox Cell Integrity Kit (Molecular Devices P/N R8213)
- CellEvent Caspase-3/7 Green Detection Reagent (Life Technologies P/N C10423)
- ScanLater Western Blot Detection System
- Anti-HSP70 mouse monoclonal antibody (R&D Systems P/N MAB1663)
- Immobilon-FL Membrane, 0.45 μm pore size (EMD Millipore P/N IPFL 000 10)
Methods
Two populations of CHO-K1 cells were prepared, healthy and stressed. The healthy cells were given regular changes of culture medium and passaged at ≤ 80% confluence, while the stressed cells were allowed to become over-confluent and had less frequent replacement of their medium.
The day before heat shock, healthy or stressed cells were seeded at 7500 cells per well in 96-well black-wall, clear-bottom tissue culture microplates for imaging and cell-based assays. For Western blot samples, cells were seeded at 225,000 cells per well in 6-well tissue culture plates.
The day after seeding, half of the cell plates were heat shocked by exposure to 45°C for 90 minutes. Immediately after heat shock, cells were imaged with the transmitted light (TL) channel of the SpectraMax MiniMax 300 imaging cytometer to look for morphological changes. At 6 hours and 24 hours after heat shock, cells were imaged again to measure proliferation and re-examine morphology. StainFree™ Technology was used to count individual cells in each well without dyes.
Cell-based assays for apoptosis and cell viability were performed to discover whether heat shock had induced apoptotic or other cell death pathways, and whether the responses differed in the healthy and stressed cell populations. The CellEvent assay was used to quantify apoptotic cells under control and heat shock conditions. Molecular Devices® EarlyTox™ Cell Integrity Assay Kit was used to distinguish and count live and dead cells. Both assays were performed 6 and 24 hours after the heat shock period.
For Western blot, cells grown and treated in 6-well plates were trypsinized, pelleted, washed and lysed in PBS with 0.5% Tween and protease inhibitors. Lysates were centrifuged, and the supernatants were assayed for protein content. 2.1 μg of cell extracts were loaded on a 4-20% gel, transferred to a PVDF membrane, and probed with mouse anti-HSP70, followed by Europium-labeled anti-mouse secondary antibody. Protein bands were detected and quantified using the SpectraMax i3 reader with ScanLater Western Blot Detection Cartridge.
Cell proliferation
After heat shock, cells were allowed to recover for 6 or 24 hours prior to imaging and other assays. At the two time points, differences in cell number were quantified using StainFree cell counting on the SpectraMax MiniMax 300 imaging cytometer. No dye was required for the software to identify and count cells. A predefined setting in the SoftMax® Pro Software was used to identify cells. A decrease in proliferation in heatshocked cells, particularly in the stressed population, was observed, and it was more pronounced after 24 hours (Figure 1).
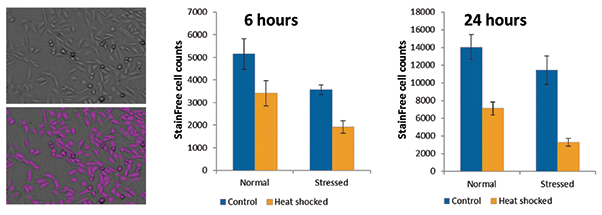
Figure 1. StainFree measurement of cell proliferation after heat shock. On the left are a transmitted light image of untreated CHO-K1 cells (top) and purple masks showing cells identified by StainFree analysis (bottom). Graphs show StainFree cell counts for normal and stressed populations of cells under control or heat shocked conditions. Cell counts were compared at 6 and 24 hours.
Apoptosis assay
At the 6 and 24 hour time points, cells were assayed for apoptosis. By 6 hours post-heat shock, stressed cells exhibited a higher percentage of apoptotic cells, but by 24 hours there were many more apoptotic cells in the stressed, heat-shocked population (Figure 2). By comparison, the non-heat-shocked cells maintained a steady and low percentage of apoptotic cells.

Figure 2. Apoptosis in control and heat-shocked cells. The left panel shows apoptotic (bright green) cells with rounded morphology, as well as several non-apoptotic cells cells with a more flattened, elongated shape. The yellow arrow points to an apoptotic cell with a high level of green fluorescence. The graphs show percent apoptosis in normal and stressed cell populations under control and heat shock conditions.
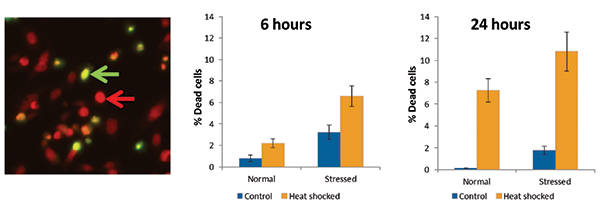
Figure 3. Quantitation of cell viability after heat shock. Heat shocked cells were imaged in the left panel (green arrow, dead cell; red arrow, live cell). Graphs show the increased percentage of dead cells upon heat shock, in both normal and stressed cell populations.
Cell viability assay
The EarlyTox Cell Integrity Kit was used to investigate whether cells were dying by other means than apoptosis. Increased cell death was observed with heat shock, particularly after 24 hours. Stressed cells had a higher overall percentage of dead cells than did the normal population. These results match reasonably well with the apoptosis result, suggesting that all or most of the cell death observed is due to apoptosis.
HSP70 induction
ScanLater Western blot analysis revealed that HSP70 was induced in response to heat shock as expected, but this response was much greater in the normal cell population than in stressed cells. With ScanLater analysis in SoftMax Pro Software, the intensity of bands on the blot was quantitated. A 48-fold increase in HSP70 was seen in the healthy cells 6 hours after heat shock, but only a 1.5-fold increase was seen in stressed cells (Figure 4). The amount of HSP70 expressed in non-heatshocked control cells was about 18-fold higher for the stressed cell population than for normal cells.
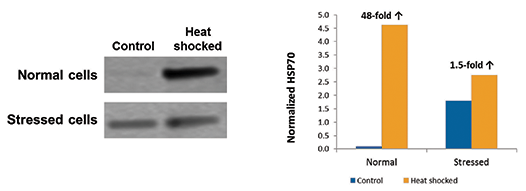
Figure 4. Western blot analysis of HSP70 expression. A Western blot for HSP70 expression in control and heat shocked cells, 6 hours after treatment, is shown in the left panel. Quantitation performed with ScanLater analysis in SoftMax Pro Software is shown in the graph. ‘Normalized HSP70’ indicates integrated density minus background, expressed in millions.
Conclusions
Using the SpectraMax i3 Multi-Mode Detection Platform, cell-based imaging assays and western blots were performed to examine the cellular response to heat shock. StainFree cell counting identified changes in proliferation with heat shock in normal and stressed cells. Apoptosis and cell viability assays provided insights into the mechanism of heat shock-induced cell death. Finally, Western blot analysis and enabled quantitation of the induction of HSP70 in control and heat shocked cells. From the results of apoptosis and cell viability assays, we hypothesize that all or most of the cell death occurring in response to heat shock was due to apoptosis. Induction of HSP70 was highest in the normal (non-stressed) cells under heat shock conditions. HSP70 was induced in stressed cells even prior to heat shock, and then induced further under heat shock. However, HSP70 in stressed cells never reached the high levels induced in healthy cells. As a result the stressed cells may have had less protection from apoptosis, leading to the higher percentage of apoptotic cells observed.
With the SpectraMax i3 system, and analysis enabled by SoftMax Pro Software, a multi-faceted investigation of cellular function that used to require multiple detection instruments and several different data analysis tools can now be performed on a single platform.
简介
许多手段可以帮助研究者来探索细胞的各 种反应过程,并且可从中获得其大量的生 物学信息,如细胞成像、基于细胞的活力 检测和增殖检测等。Western Blot(即蛋 白质免疫印迹法)不仅可用于观察蛋白质 表达情况的变化。通常情况下我们需要多 台仪器协同工作才能够获得足够多的信 息,当然整个过程我们也不得不需要掌握 更多种不同的软件。
此篇应用文章我们展示出如何仅利用一台 仪器,即SpectraMax® i3多功能检测平台 就可以完成对许多细胞相应参数的收集和 处理,我们用热应激反应作例来展示不同 检测方式特点,包括成像和Western Blot 功能,多重手段方便我们观察细胞内不同 反应过程带来的不同变化。
众所周知正常细胞长时间暴露在高温环境 下会引起细胞凋亡,同样也会提高细胞 HSP70(Heat Shock Protein)的表达 量。我们将CHO-K1细胞置于两种不同的 生长环境下,即健康环境和压力环境,首 先将两种环境下的细胞经过热激处理以 后,我们利用SpectraMax® i3多功能检 测平台的细胞成像功能去检测其细胞增 殖、活力和凋亡的情况。我们也可利用 ScanLaterTM Western Blot检测系统获 得的数据经分析后发现,HSP70表达量与 热激反应成正相关性。
材料
- CHO-K1细胞(ATCC P/N CCL-61)
- 细胞培养基(Ham’s F12中加入10%胎 牛血清和1%的双抗)
- 黑色,底透细胞培养微孔板(Corning P/N 3603)
- SpectraMax i3微孔板读板机
- SpectraMax MinMaxTM 300细胞成像 系统
- EarlyTox Cell Integrity试剂(Molecular Devices P/N R8213)
- CellEvent Caspase-3/7绿色检测试剂 (Life Technology P/N C10423)
- ScanLater Westen Blot检测系统
- ScanLater抗鼠检测试剂盒-铕元素标记羊抗鼠的二抗
- ScanLater Western Blot检测卡盒
- 抗HSP-70鼠源单抗(R&D检测系统 P/N MAB1663)
- Immobilon-FL膜,0.45µm孔径 (EMD Millipore P/N IPFL 00010)
方法
预先处理两份CHO-K1细胞,分别将其处于健康环境和压力环境。健康细胞为定期更换培养基而且传代过程保证其汇合度不高于80%,然而压力环境下的细胞不经常更换培养基且汇合度较高时才进行传代处理。
进行热激反应前一天,我们分别将健康环 境下培养细胞和压力环境下培养细胞铺在 黑色底透96孔板中,密度为7500细胞/ 孔,采用黑色底透的细胞培养微孔板的目 的是便于细胞成像和细胞功能学实验。针 对于Western Blot样品准备,预先以 225,000细胞/每孔的密度铺于6孔细胞培 养板中。第二天,我们将一半的细胞进行 热激处理,即置于45°C环境下90分钟, 热激处理后,将细胞立刻放置于 SpectraMax MiniMax 300细胞成像系 统下,并利用其透射光通道观察细胞形态 的变化。经热激反应6小时和24小时后, 利用系统成像功能再次观察 细胞形态学变 化以及增殖情况。StainFree™ 技术(免 标记分析技术)无需染料分子既可以检测 每孔中细胞的数目。
利用细胞功能学实验分析细胞凋亡和细胞 活力情况,探索热激反应能否诱导凋亡或 者其他细胞死亡通路,观察健康环境下培 养的细胞和压力环境下培养的细胞是否有 不同的表现。CellEvent试剂用于热激 处 理 后 细 胞 凋 亡 的 检 测 , M o l e c u l a r Devices® EarlyTox™ Cell Integrity试剂 盒可用于区分活细胞与死细胞。两种检测 分别在其热激反应后的第6小时和第24小 时。
对于Western Blot检测,细胞置于6孔板 中进行培养,我们用胰酶消化细胞后,用 含有0.5%Tween和蛋白酶抑制剂的PBS 缓冲液来清洗和溶解细胞,然后离心,上 清液进行蛋白定量检测,我们将2.1ug细 胞提取物,上样于4-20%浓度的胶中,然 后再将蛋白转移至PVDF膜上,一抗采用 鼠源的抗HSP-70抗体,二抗采用铕元素 标记抗鼠的二抗,我们利用SpectraMax i3 检测平台组合ScanLater Western Blot 检测卡盒对蛋白条带进行定量检测。
细胞增殖
热激反应后,在进行细胞成像和其他实验 前,我们先将细胞经过6小时和24小时恢 复性生长。然后分别在这两个时间点,利 用StainFree技术在SpectraMax MiniMax 300细胞成像系统上对细胞进行计数分 析,无需染料既可以利用软件优势识别细 胞并对其进行计数。利用SoftMax® Pro软 件中预置程序识别细胞,分析热激反应后 细胞增殖下降的情况,尤其是压力环境下 处理的细胞,观察发现,24小时候后细胞 增殖下降的情况更加明显(图一)。

图一:StainFree技术分析热激后细胞增殖情况。 左侧区域(上图)显示的为未经处理CHO-K1细胞在透射光成像结果,(下图)紫色伪彩覆盖的细胞为StainFree技术分析后识别的细胞。柱状图显示为StainFree技术对正常细胞和压力细胞质控组和热激处理组进行计数,分别比较其在6小时和24小时的细胞计数结果。
凋亡实验
在6小时和24小时的两个时间点,对细胞凋亡情况进行检测,热激反应的第6小时,压力环境下的细胞凋亡百分率会更高,随后第24小时我们发现压力环境下培养的细胞其凋亡情况更加明显(图二)。比较后我们发现,未经过热激处理的细胞比较稳定且凋亡细胞比率较低。

图二:质控组和热激组细胞凋亡情况。 左侧区域图显示细胞凋亡情况,细胞形态为圆形(亮绿色),若干个未凋亡细胞扁平的铺于微孔板底部,呈现细长形状。黄色箭头为凋亡细胞为高亮绿色,柱形图显示在质控和热激条件下正常细胞和压力细胞凋亡比率。

图三:热激后细胞活力检测。 左侧区域为热激细胞成像结果(绿色箭头为死亡细胞,红色箭头为活细胞),柱形图显示在正常条件和压力条件下热激处理后死亡细胞增加比率。
细胞活力实验
利用EarlyTox Cell Integrity试剂盒来检测 细胞是否死亡,热激反应后我们发现死亡 细胞数目明显增加,尤其是在第24小时 后,压力环境下的细胞比健康环境下的细 胞死亡比率更高。这个结果与凋亡检测结 果相一致,可以说明全部或者大部分的细 胞死亡结果是由于细胞凋亡所引起的。
HSP70诱导
Scanlater Western Blot分析后揭示的结 果如预期,经热激反应后HSP70诱导情况 为在正常健康环境下处理的细胞的表达量 更大。通过SoftMax 软件和Scanlater系 统,我们可以针对条带强度进行定量分 析,热激反应6小时后健康环境下的细胞 HSP70表达量增加了48倍,但是在压力 环境下其仅仅增加了1.5倍(图四)。未 经过热激处理的压力环境下细胞其HSP70 表达量较健康环境下的细胞高出18倍。

图四:Western Blot分析HSP70表达情况。 。左侧区域显示的是利用Western Blot技术分析处理6 小时后的质控组细胞和热激组细胞HSP70表达情况,柱形图显示为利用ScanLater和SoftMax Pro 软件定量分析的结果,标准化HSP70结果,即条带信号强度减去背景信号。
结论
利用SpectraMax i3多功能检测平台的细 胞成像和Western Blots功能来研究热激 处理后细胞的反应及变化情况。StainFree细胞计数功能来检测正常及压力细胞 热激后增殖情况的变化,细胞凋亡和活力 实验可以帮助我们了解热激诱导细胞死亡 的机制。最后,利用其Western Blot功能 来定量检测其热激细胞和质控细胞(未经 过热激处理的细胞)诱导的HSP70含量。 从细胞凋亡和细胞活力实验结果,我们假 设全部或者大部分细胞的死亡是由于热激 处理后引起的细胞凋亡。我们发现正常培 养的细胞(无压力环境培养的细胞)热应 激处理后诱导产生的HSP70量最高。而压 力环境下培养的细胞在进行热激反应以前 就已经表达HSP70,然后在热激反应后会 进一步提高HSP70表达量。
然而,压力环境培养下的细胞产生的HSP70量远低于健康环境下培养的细胞,结果揭示出压力环境下培养的细胞在凋亡过程中的保护机制要更低,所以可以观察到细胞凋亡比率会更高。
我们现在仅需利用 SpectraMax i3检测平 台和SoftMax Pro分析软件,就能够采用 多种方式来探究细胞功能,而传统上实现 这些检测会需要多台不同仪器和多款不同 软件的配合使用才能够完成。