TRF / TR-FRET (HTRF) Overview
Time-Resolved Fluorescence (TRF)
Fluorescence intensity (FI) measurements use standard fluorophores like fluorescein, whose emission is short-lived, on the order of nanoseconds. Excitation of the sample and measurement of emission occur simultaneously. Although microplate readers are very good at screening out excitation light from the emission measurement, that excitation light, along with short-lived light emitted by materials in the well or sample, often contributes to high background.
Time-resolved fluorescence (TRF) reduces background by using a lanthanide fluorophore, such as europium or terbium, that emits long-lived fluorescence. This long-lived fluorescence lasts for milliseconds, so excitation of the fluorophore by a pulsed light source (e.g., a flash lamp), followed by a delay and then signal measurement (‘counting window’), allows short-lived fluorescence (lasting only for nanoseconds) to subside before a measurement is made. Assays using time-resolved fluorescence offer dramatically increased signal-to-noise ratios. The most frequently used lanthanides are europium, terbium and samarium. These are commonly used as chelate or cryptate complexes that enable good signal intensity and stability.
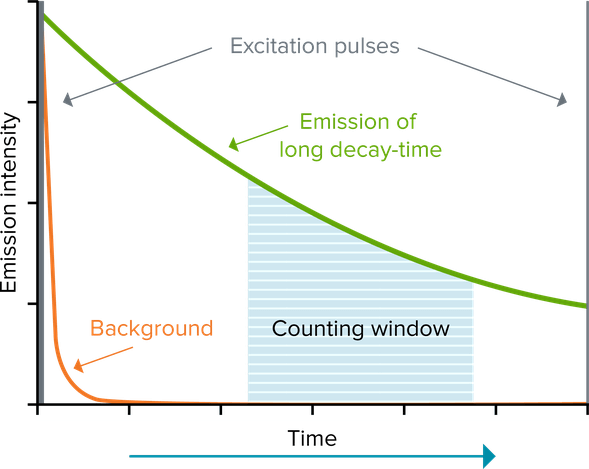
Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET)

no FRET FRET
Donor and acceptor are distant Donor and acceptor are inclose proximity
TR-FRET combines the time-resolved (TR) measurement of fluorescence with fluorescence resonance energy transfer (FRET) technology. In FRET assays, biomolecules (e.g., proteins) are labeled with donor and acceptor fluorophores. When the biomolecules interact, donor and acceptor fluorophores are brought close together. Now, when the donor is excited, it can transfer its emission energy to the acceptor, which in turn emits fluorescence at a specific wavelength. Acceptor and donor fluorescence emissions have different wavelengths that can be distinguished from each other by a microplate reader, enabling quantitation of the biomolecular interaction.
Using lanthanide fluorophores, which have long-lived fluorescence emission, as donors, TR-FRET assays take advantage of the time-resolved measurement of fluorescence to eliminate short-lived background fluorescence. In a TR-FRET assay, thanks to the donor fluorophore’s long-lived emission, excitation and emission of both donor and acceptor fluorophores can also be measured after short-lived background fluorescence has abated.
Homogeneous Time-Resolved Fluorescence (HTRF)
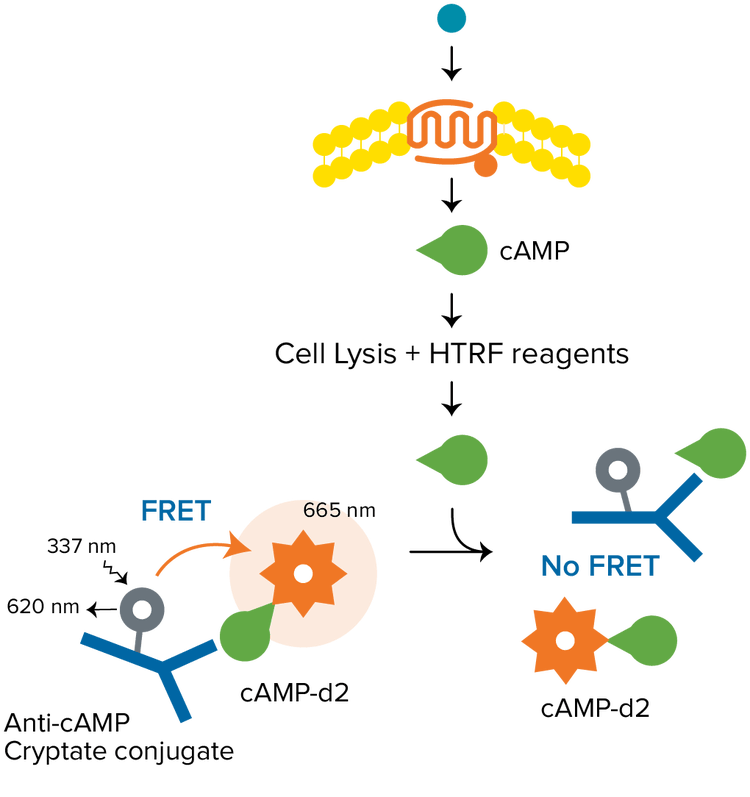
HTRF is a versatile TR-FRET technology developed by Cisbio for detecting biomolecular interactions. A typical HTRF assay uses a europium cryptate as the donor, and the organic fluorophore d2 as the acceptor. The donor and acceptor can be used to label a variety of biomolecules, for applications including epigenetics, biomarker quantification, GPCR signaling, and much more. HTRF assays require a microplate reader with TRF detection mode that is certified HTRF-compatible by Cisbio.
Advantages and Considerations
A key benefit of TRF and TR-FRET detection includes decreased background and increased signal-to-noise ratio compared to standard fluorescence, resulting in higher sensitivity. In addition, the assay has a robust mix-and-read format that does not require any washing. This, along with the assay’s stability, makes it easy to automate and miniaturize for screening applications.
Applications and Assays
TRF and TR-FRET (HTRF) assays enable the analysis of molecular interactions in biochemical processes and are widely used to study kinase assays, cellular signaling pathways, protein-protein interactions, DNA-protein interactions, cell cytotoxicity and receptor-ligand binding.
Example assays for TR-FRET are outlined in the table to the right, along with several time-resolved fluorescence resources below.

