
Fluorescence Polarization (FP)
Fluorescence Polarization (FP)
Fluorescence polarization (FP) is a technique that is widely used to monitor binding events in solution. It can be used to assess biomolecular interactions, including protein-antibody binding and DNA hybridization, as well as enzyme activity, and it has been adapted to basic research as well as high-throughput screening.
A small, fluorescently labeled molecule (tracer) that is excited with plane-polarized light emits mostly depolarized light because the tracer tumbles rapidly during the time between excitation and emission. However, when the tracer binds a much larger molecule, it rotates more slowly, and the emitted light remains largely polarized.
Fluorescence polarization G factor
Polarization, expressed in units of milli P, or mP, is calculated from the measurements of perpendicular (Iperp) and parallel (Ipara) fluorescence intensity values detected relative to the direction of the polarized excitation light (see the formula below). The G factor (G) is used to correct for the effects of optical components like filters, polarizers, and monochromators, which can affect polarization values.

Unbound tracer
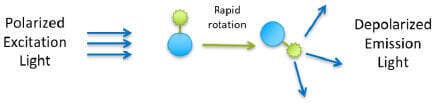
Tracer bound to a larger molecule
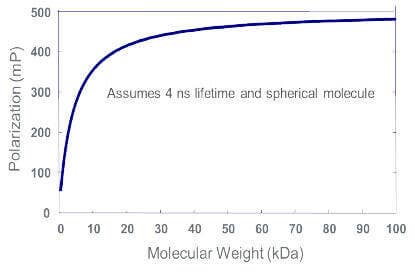
The larger the fluorescently labeled molecule, or the molecule to which tracer is bound, the larger the mP value.
FP advantages over other binding assays
FP can be employed to study receptor-ligand interactions, protein-DNA interactions, proteolysis, membrane fluidity, enzyme assays, and more. FP assays have been used successfully to investigate a wide variety of targets including kinases, phosphatases, proteases, G protein–coupled receptors (GPCRs), and nuclear receptors.
The following benefits make FP assays especially amenable to high-throughput screening:
- Homogeneous (no wash steps required)
- Non-radioactive
- Ratiometric (single wavelength)
- Miniaturizable
IMAP phosphodiesterase assays on SpectraMax Multi-Mode Microplate Readers
IMAP® Technology from Molecular Devices enables rapid, homogeneous, and non-radioactive assay of kinases, phosphatases, and phosphodiesterases and is suited for both assay development and high-throughput screening. IMAP assays are based on binding of phosphate to immobilized metal coordination complexes on nanoparticles. When IMAP binding entities bind to phosphorylated substrate, molecular motion of the peptide is altered, and fluorescence polarization (FP) for the fluorescent label attached to the peptide increases (Figure 1, left). In a TR-FRET version of the assay, the inclusion of a Terbium (Tb) donor enables a fluorescent energy transfer to occur when phosphorylated substrate is present (Figure 1, right). This assay is detected in a time-resolved mode, which virtually eliminates fluorescence interference from assay components or compounds in a screen. TR-FRET also offers flexibility in substrate size and concentration.
Cyclic nucleotide phosphodiesterases (PDEs) are a group of enzymes that degrade the phosphodiester bond of cAMP and cGMP, second messengers that are involved in a variety of biological processes. They have emerged as a key class of druggable targets due to their clinical significance in areas including heart disease, dementia, depression, and others. Here, we demonstrate how PDE enzyme dilution and inhibition curves are performed with IMAP Technology using the SpectraMax® Multi-Mode Microplate Readers with SoftMax® Pro Software.
IMAP FP and TR-FRET phosphodiesterase assay principle
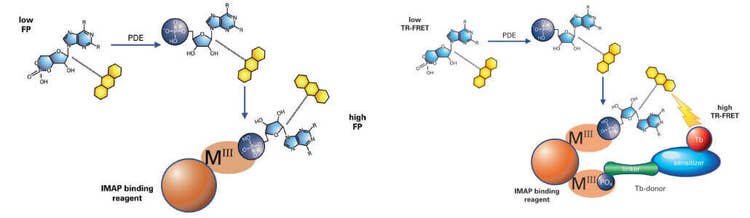
Figure 1: A phosphodiesterase reaction is performed using a fluorescent-labeled substrate. Binding Solution containing large M(III)-based nanoparticles is then added. In the FP readout (left), the small phosphorylated fluorescent substrate binds to the large nanoparticles, which reduces the rotational speed of the substrate and thus increases its fluorescence polarization. In the TR-FRET readout (right), phosphorylated substrate and Tb donor both bind to the nanoparticle, bringing the Tb donor in close proximity to the fluorescein acceptor on the substrate and enabling FRET.
Streamline the workflow for measuring IgG in cell line development
The measurement of IgG production is a crucial step at many stages in the development and manufacturing of monoclonal antibodies. Commonly used methods for IgG quantitation require either special instrumentation and skilled personnel, such as HPLC and surface interferometry, or time-consuming assays like ELISA (enzyme-linked immunosorbent assay). Although the ELISA is a well-established method for protein quantitation, it is a lengthy, multi-step process. Here, we introduce the use of the Valita®TITER assay from Valitacell for measuring IgG titers during the antibody development and manufacturing process.
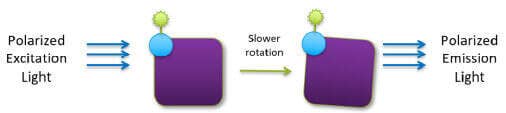
The ValitaTITER assay is based on detection of IgG Fc interactions with Protein G using fluorescence polarization (FP) technology. Each well of a 96- well microtiter plate is coated with a fluorescently labeled IgG-binding peptide, Protein G. When samples are added to the wells, the Protein G molecules are resuspended and binding occurs. The rate of molecular motion of Protein G slows down when it is bound to antibodies, resulting in an increase of the FP value (Figure 2).
ValitaTITER assay principle using the FP technology
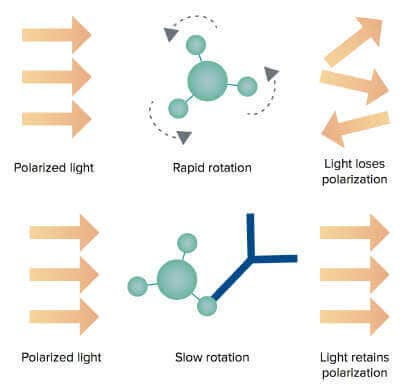
Figure 2: Free Protein G molecules are smaller and rotate faster in the buffer solution. As a result, the polarized excitation light loses polarization (top). When Protein G molecules bind to the antibodies in the samples, the rotation of the larger complex slows down resulting in increase of the FP values (bottom).