
Application Note
Assaying cardiotoxicity with ImageXpress high-content screening systems
- Assess cytotoxic effect of compound derived from induced pluripotent stem cells
- Expedite drug discovery process by reducing unforeseen cardiac side effects late in clinical trials
- Implement high-throughput screening for multiplexed, cell-based assays
Introduction
The discovery and development of novel drugs is a difficult and expensive process due to lack of tools and techniques to accurately reproduce the human physiological response in the laboratory. Off-target cardiotoxicity remains a significant cause of pre- and post-approval safety-based drug attrition because of the disconnect between the behavior of cultured immortalized cells and in vivo animal models to accurately simulate human cardiac disease states.Using high-content imaging systems, like the ImageXpress® Micro system, it is possible to assess the cytotoxic effect of compounds on pertinent human cardiac cells derived from induced pluripotent stem cells.
Analyze gross cytotoxicity using live/dead assay
Membrane-permeable Calcein AM is cleaved by esterases in live cells to produce green fluorescence in the cytoplasm. In dead cells, the cell membrane is compromised, allowing the dye, ethidium homodimer, to enter and stain the nucleus (Figure 1).
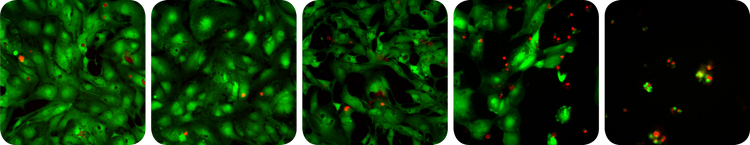
***Figure 1. Cytotoxicity assay. *Cardiomyocytes exposed to increasing levels of a toxic compound (left to right). Live cells exhibit green fluorescence and an increasing incidence of red stained dead cells is evident at higher doses.
Cardiomyocytes derived from human induced pluripotent stem cells (iPsc, iCell Cardiomyocytes from Cellular Dynamics Intl.) were incubated with varying concentrations of drugs which were analyzed for cadiotoxicity using high-content imaging (Figure 2) with the data plotted normalized to control (Figure 3). Valinomycin (columns 9 and 10) were highly cardiotoxic even at the lowest levels tested.
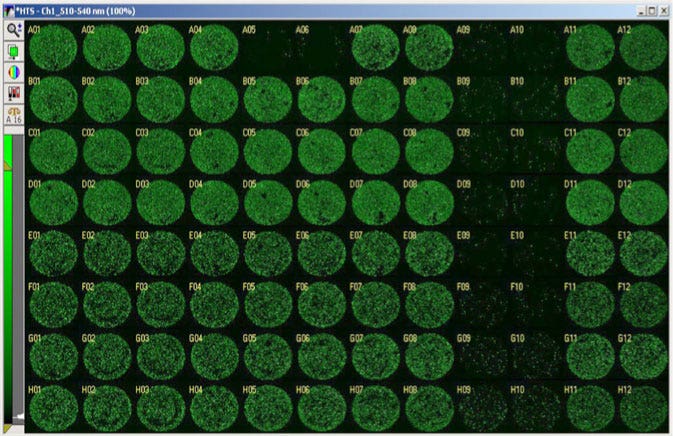
Figure 2. Whole well images of toxicity assay. Cardiomyocytes screened against an 8 point doseresponse of 5 different compounds tested in duplicate. The compound Valinomycin, in columns 9 and 10, was toxic even at the lowest dose tested (row H).
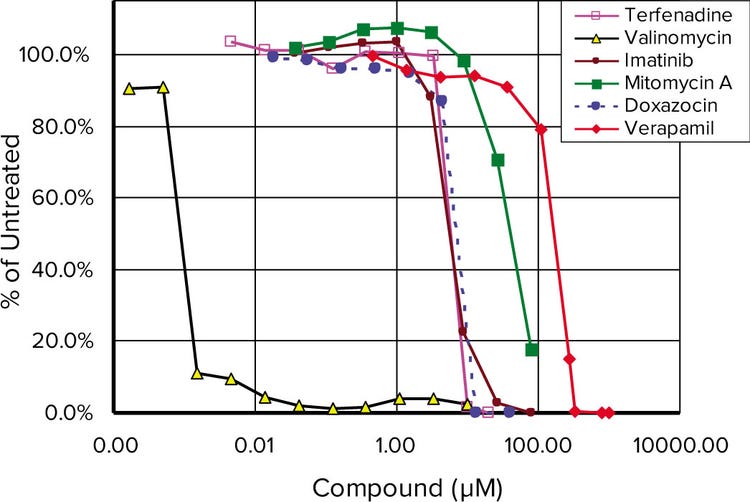
Figure 3. Toxicity dose response curves. . Normalized results of a live/dead assay run on 6 different compounds after 48 hours of drug treatment.
Monitor mitochondria membrane depolarization with JC-10
JC-10 aggregates in the mitochondria of healthy cells to produce an orange/red emission at 570 nm. Some compounds cause immediate toxic effects by disrupting the membrane potential of the mitochondria. If the mitochondrial membrane is compromised, the JC-10 monomers disperse in the cytoplasm where they emit at 530 nm. Quantitative detection of the mitochondrial membrane change is possible by monitoring the fluorescence intensity ratio at 570 nm/530 nm or simply by quantitating the amount of orange mitochondria detected per cell.
The ImageXpress Micro systems are a fully integrated hardware and software system for automated acquisition and analysis of images for high-throughput cell-based cytotoxicity testing. When configured with optional environmental control, we could monitor living cell responses or kinetic reactions in real-time for several days. In this study, we incubated cardiomyocytes and applied increasing concentrations of Antimycin A. After 90 minutes of incubation, we quantitated the cardiotoxicity (Figure 4). The saved images could be reviewed at any time and further analyzed with one of the MetaXpress® software application modules.
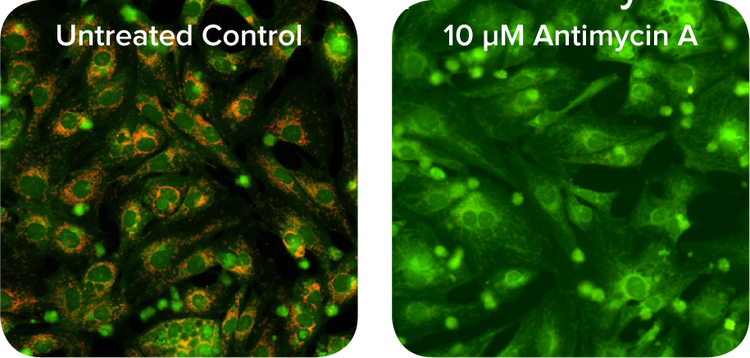
Figure 4. JC-10 mitochondrial membrane potential assay. Increasing concentrations of Antimycin A cause more depolarization of mitochondrial membrane leading to a lower ratio of red-stained mitochondria to green fluorescent cytoplasm. Live cell images were acquired at 20X using ImageXpress Micro System with environmental control. I
Summary
The ImageXpress Micro system is a powerful system for analysis of in vivo drug cardiotoxicity using cardiomyocytes derived from human induced pluripotent stem cells in both the whole cell cytotoxicity and mitochondria membrane depolarization assays. The system provides pharmaceutical scientists with critical information on compound cytotoxicity early in the drug discovery process to help reduce unforeseen cardiac side effects late in clinical trials.
简介
由于缺乏设备和技术手段在实验室中精确 再现人体内的生理反应,导致新药的研发 过程难度大、成本高。体外细胞实验和体 内动物实验在精确模拟人类心脏病病理状 态时存在很大差异,因此确定脱靶效应对 心脏的毒性仍然是基于消耗量所进行的前 期后期药物安全性评价的重要目的。利用 高内涵成像系统(如:ImageXpress Micro System)可以对诱导多能干细胞(iPSC)来 源的人类心肌细胞在化合物处理后产生的 细胞毒性进行准确评价。
利用活/死细胞检测法分析总细胞毒性
可透膜的钙黄绿素进入活细胞后经酯酶分 解在胞质中产生绿色荧光。死细胞膜通透 性增加, 染料溴乙非啶豪莫二聚体 进入细 胞并对细胞核进行染色(图1)。

图 1. 细胞毒性检测. 用毒性化合物处理心肌细胞(化合物浓度从左至右逐渐增大). 活细胞标记为绿色荧光,随着化合物浓度的增加,标记为红色荧光的死 细胞比例随之增加
将诱导多能干细胞(iPSC)来源的心肌细胞经不同浓度的药物处理,用高内涵对样本成像(图2)并结合对照组进行心肌细胞毒性分析(图3)。缬氨霉素(图2第9、10列)在低浓度下也表现出较高的心肌细胞毒性。

图 2. 细胞毒性检测. 用毒性化合物处理心肌细胞(化合物浓度从左至右逐渐增大). 活细胞标记为绿色 荧光,随着化合物浓度的增加,标记为红色荧光的死细胞比例随之增加

图3. 毒性剂量曲线. 6中化合物处理48小时后的细胞,用活/死细胞应用模块进行分析并作图
利用JC-10监测线粒体膜去极化
JC-10聚集在活细胞线粒体中,发出570 nm 的橙红色荧光。部分化合物通过干扰线粒 体膜电位,产生直接毒性作用。一旦线粒 体膜通透性增大,JC-10单体便分散于细 胞质当中发出530 nm荧光. 通过检测每个 细胞中橙色线粒体数量或细胞中570 nm/ 530nm的荧光强度的比值即可定量检测线 粒体膜变化。
ImageXpress Micro 系统将硬件、软件整 合在一起,在高通量细胞毒性评价过程中 对图片采集和分析。搭配环控组件后可以 对活细胞进行实时观察和动力学分析。本 实例中,心肌细胞与一定浓度梯度的抗霉 素A孵育,90分钟后对心肌细胞毒性进行 量化(图4)。采集的图片可以随时调取或用 MetaXpress®软件的应用模块对图片进行 深度分析。

图4. JC-10 线粒体膜电位分析. 增加抗霉素浓度,线粒体膜去极化作用增加,红色荧光(线粒体)与绿色 荧光(细胞质)的比值降低. 在高内涵环境控制模式下使用20X物镜拍摄上图
结论
ImageXpress Micro 高内涵成像系统是一 种高效强大的成像分析系统,可从全细胞 水平和线粒体膜去极化两个层次分析、评 价诱导多能干细胞来源的心肌细胞的体内 药物毒性结果。高内涵成像系统可以为药 物学家提供早期药物研发所需的关键信 息,并减少临床试验中产生的心脏方面的 不确定影响。