
Stable Cell Line Generation for Vaccine Production
Streamline your workflow for stable cell line generation
Stable cell line generation is a cornerstone of biopharmaceutical research, particularly in vaccine development and therapeutic protein production. This critical process involves engineering a clonally derived cell population that maintains the stable expression of desirable phenotypes, such as high-yield recombinant protein production. By systematically optimizing this workflow, researchers can accelerate development timelines, enhance reproducibility, and ensure consistent product quality.
Our comprehensive guide outlines the essential steps of the stable cell line generation process, from stable transfection to titer screening. Whether you're developing a cell line for antibody production or other biotherapeutics, understanding and refining each step is vital for success.

Workflow for stable cell line generation
Step 1: Stable transfection
The process begins with the introduction of foreign DNA encoding the recombinant protein of interest into a host cell, known as transfection. While most cells express the protein transiently for a short duration, a subset integrates the DNA into their genome, resulting in stably transfected cells. These cells are selected to advance to subsequent steps due to their prolonged protein expression capabilities.
Step 2: Pool enrichment
After stable transfection, researchers enrich the cell population by isolating those that maintain high expression levels. This step often utilizes a selectable marker like Green Fluorescent Protein (GFP) to differentiate stably transfected cells. GFP fluorescence intensity correlates strongly with recombinant protein levels, making it an effective tool for enriching high-producing cell pools.
Step 3: Single cell isolation
Pool enrichment typically yields a heterogenous cell population with varying protein expression levels. To achieve a genetically uniform population, single-cell isolation is essential. This ensures clonality, which is critical for regulatory compliance and reproducible results. Researchers use techniques like limiting dilution, flow cytometry, or advanced imaging systems to isolate single cells.
Step 4: Monoclonality verification and cell growth
Verification of monoclonality ensures that the derived colonies originate from a single cell. Advanced cell imaging technologies document this stage, providing evidence for regulatory submissions. Following isolation, cells are monitored for growth and productivity, ensuring they meet the required standards.
Step 5: Titer and critical quality attribute (CQA) screening
The final step involves assessing the productivity and quality of the stable cell line. Researchers measure protein titer, which determines the concentration of recombinant protein produced. They also evaluate critical quality attributes (CQA), such as glycosylation patterns or antibody structure, ensuring the final product meets therapeutic standards.
COVID-19 related research solutions
Accelerate your COVID-19 research
Molecular Devices is committed to supporting scientists that are researching COVID-19 cellular response and vaccine development by offering technology and solutions that you can deploy rapidly.
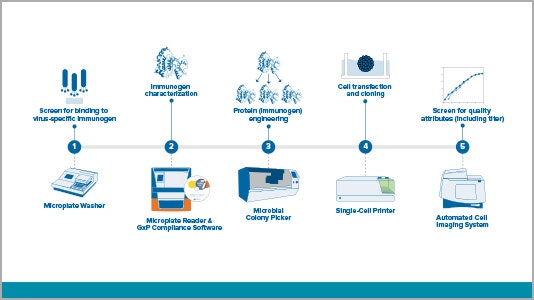
Workflow solutions for vaccine development
View a variety of virus-related workflows – from antigent/immunogen and antibody discovery to stable cell line development.
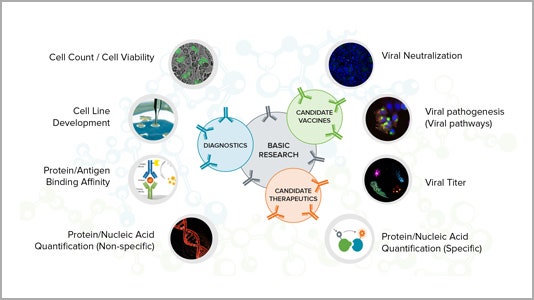
Applications for critical research
Learn more about how our technology can help your research of COVID-19 and other infectious diseases for vaccine development, therapeutics and diagnostics.
Resources related to COVID-19 cellular responses and vaccine development
Systems to accelerate your COVID-19 cellular response and vaccine development
We have validated and compliant laboratory solutions including microplate readers, microplate washers, biopharma and cellular imaging systems to meet your research needs.
