A complete solution for the automated screening and objective selection of high-value clones across diverse cell types.
The ClonePix® 2 Mammalian Colony Picker is a fully automated system for selecting high-value clones used in antibody discovery and cell line development. Screen more clones in less time with monoclonal verification on day zero, then screen and identify for highest producers in weeks, not months.
Hybridomas, CHO cells, stem cells and other cell types are imaged and selected based on user-defined parameters. Plate handling, barcode reading, and picking are fully integrated, and all data, including images, are saved for downstream analysis. The picker increases the probability of finding optimally produced cell lines and significantly reduces time and labor.

Automate antibody discovery and cell line development workflows
ClonePix 2 picker is 10x faster than labor-intensive limiting dilution and FACS. Our sophisticated software and integrated robotics enable a screening speed of > 10,000 clones per day.

Select cells with desirable attributes with monoclonality assurance from day 0
Easily screen and select clones based on protein productivity, antigen-specificity, cell viability, and expression levels of tagged recombinant proteins.

Increase the probability of identifying high-value clones while eliminating or recovering unstable clones early
Picking accuracy < 1 mm. Robotic picking reduces the risk of colony disturbance. Images of picked clones are stored with data.

ClonePix 2
Features

Multiple detection methods
White light identifies and measures clone morphology, size and proximity. Fluorescence indicates expression level and/or specificity. Up to five fluorescent filters are available for multiplexing.

Sterility maintenance
A host of sterility features and options including a UV light process for sanitizing the interior of the instrument, as well as pin washing and halogen drying are standard.

Integrated plate storage
Includes two storage stacks for source and destination plates, each with a capacity of 10 plates.

Discrete colony formation
Semi-solid CloneMediaTM encourages single cells to grow into discreet colonies, and allows for ease of plating. The media allows for a higher density of clones to be screened.

Animal-free media and reagents
Chemically defined and animal-free, CloneMedia cell-culture media, is optimized to increase productivity and aid in visualizing secreted antibodies when used with the CloneDetectTM detection agent.

Custom automation options*
The Advanced Workflow Engineering Solutions Team can customize the monoclonality system and offer added services such as integrated verification of monoclonality.
Redefine clone screening and selection with transformative cell line development workflows
Empower your team with data analysis that automatically generates a map of clones and their secretion levels from a series of images generated in situ. The ClonePix can also be customized to include image-based monoclonality assurance on day zero.* This means your team can screen in one round and then pick for the highest produces in weeks, not months.
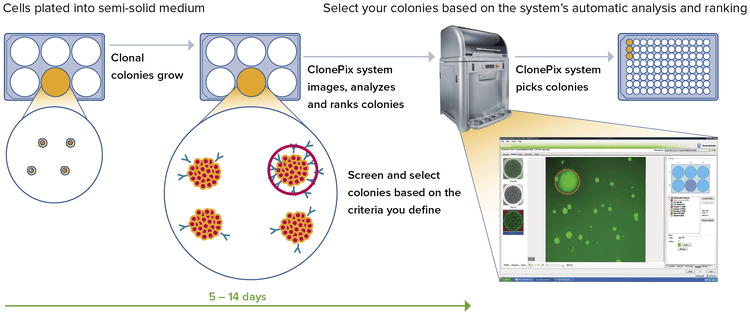
Data analysis and tracking—reveal stable clones faster

Data Analysis
- Automatically generate a 2D map of clones and their secretion levels from a series of images generated in situ
- Screen and select colonies based on:
- Size, roundness, and proximity to neighbors
- Ranking according to fluorescence levels
- Closely placed colonies ignored via user-controlled “proximity” software setting
Data Tracking
All relevant data associated with each colony (including images taken before and after picking along with their picking coordinates) are automatically saved for review and downstream analysis.
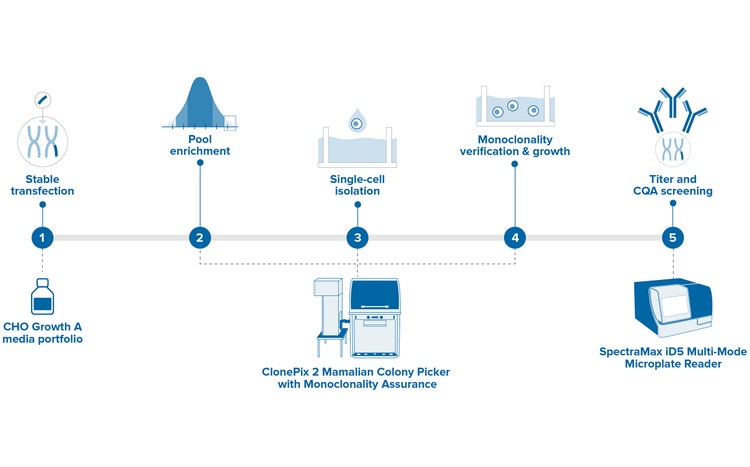
Enhanced for Monoclonality Verification*
Offering high-resolution single-cell imaging capability on day 0
The enhanced ClonePix 2 system can automatically screen and pick clones that are both high-producing and monoclonal—all in one system. Screen more clones in less time with monoclonal verification on day zero, then screen and pick the highest producers in less than two weeks.
Key Benefits:
- Rapid Monoclonal Verification: Achieve image-based clonality verification on day zero, reducing screening rounds from two to one.
- Advanced Imaging Capabilities: Rapid Z-stack acquisition allows detection of single cells throughout the medium volume, not just a single focal plane.
- Streamlined Workflow: Simplified process from single-cell identification to productivity screening, enhancing efficiency with the all-in-one system.
- Higher Throughput: Process up to 12,000 colonies on just 10 plates, significantly increasing laboratory productivity.
- Cost-Effective Operations: Minimize operational costs through reduced manual labor, decreased media volume requirements, and enhanced processing speed.
Enhanced for stem cell applications*
Identify desirable, clonal stem cell colonies for high-throughput colony screening and picking
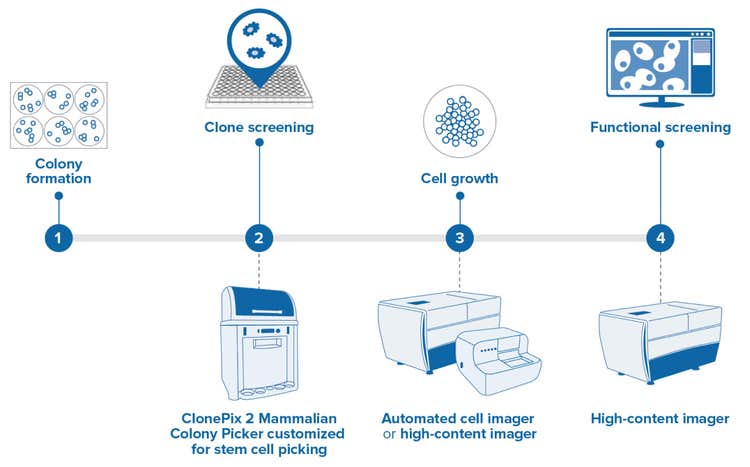

High-resolution imaging identifies desirable, clonal stem cell colonies for high-throughput colony screening and picking. Specialized picking pins allow the gentle transfer of adherent, feeder-free cells to high-density plates for clonal expansion and downstream analysis.
- Colony formation - Individual stem cells plated at a low density in 6-well plates divide and develop into colonies. Plating density is kept low to ensure that colonies are derived from a single parent cell.
- Clone screening - Clonally-derived stem cell colonies are identified by their desirable morphological characteristics and are picked from the low-density 6-well plates into higher density 96-well plates to carry forward with the screening. The ClonePix® 2 Mammalian Colony Picker can be customized with high-resolution optics and stem cell-specific pins, allowing this established technology to be leveraged in stem cell workflows.
- Cell growth - Cell growth is determined by monitoring cell division over a given time using label-free imaging.
- Functional screening - In addition to monitoring growth, functional cell-based assays can be performed. This can include assays assessing differentiation potential, pluripotency, ability to form 3D organoids, and other desirable traits.
ClonePix Kits
Additionally, consider integrating our specialized ClonePix Reagent Kits to further enhance your system's capabilities. These kits provide optimized media for CHO cell line development and convert existing media to semi-solid form, ensuring colony integrity and enabling fast screening and high-value clone selection.

*Price, time to deliver, and specifications will vary based on mutually agreed technical requirements. Solution requirements may cause an adjustment to standard performance.
Custom solutions are subject to Molecular Devices Custom Products Purchase Terms.
FAQs
What is the ClonePix 2 Mammalian Colony Picker?
The ClonePix 2 Mammalian Colony Picker is a cutting-edge technology developed by Molecular Devices for efficient and automated selection of mammalian colonies. It streamlines the process of clone screening, enabling researchers to quickly identify and isolate high-value clones for downstream applications.
How does the ClonePix 2 Mammalian Colony Picker work?
The ClonePix 2 Mammalian Colony Picker uses advanced imaging and robotic technology to analyze and pick individual mammalian colonies. It captures high-resolution images of the colonies, and based on predefined criteria, it selects and picks the desired clones using a gentle and precise picking mechanism. This automated process saves time and reduces human error associated with manual colony picking.
What are the key features of the ClonePix 2 Mammalian Colony Picker?
The ClonePix 2 Mammalian Colony Picker offers several key features, including high-speed imaging, customizable colony picking parameters, flexible plate formats, and a user-friendly interface. It also provides colony-level data analysis, enabling researchers to make informed decisions during clone selection.
What are the benefits of using the ClonePix 2 Mammalian Colony Picker?
The ClonePix 2 Mammalian Colony Picker offers numerous benefits. It improves productivity by significantly reducing the time and effort required for clone screening. It enhances accuracy and reproducibility by eliminating manual handling errors. Additionally, it enables researchers to identify rare and valuable clones, leading to higher success rates in downstream applications.
What applications can the ClonePix 2 Mammalian Colony Picker be used for?
The ClonePix 2 Mammalian Colony Picker is widely used in various applications such as antibody discovery, cell line development, protein expression optimization, and genetic engineering. It is a versatile tool that accelerates research and development in pharmaceutical, biotechnology, and academic laboratories.
What does the ClonePix 2 Mammalian Colony Picker do that sets it apart from other colony screening methods?
The ClonePix 2 Mammalian Colony Picker revolutionizes colony screening by combining advanced imaging technology with automated colony picking. Unlike traditional manual methods, it offers high throughput, precision, and reproducibility. This powerful combination saves time and resources while improving the accuracy of clone selection.
In what workflows can the ClonePix 2 Mammalian Colony Picker be incorporated?
The ClonePix 2 Mammalian Colony Picker can be seamlessly integrated into various workflows, including monoclonal antibody production, cell line development, CRISPR/Cas9 genome editing, and recombinant protein expression. Its versatility allows researchers to optimize these workflows by efficiently selecting the most promising clones for downstream applications.
How can the ClonePix 2 Mammalian Colony Picker optimize scientific research?
The ClonePix 2 Mammalian Colony Picker significantly enhances scientific research by streamlining the clone screening process. It enables researchers to identify and select high-value clones quickly, ensuring a higher success rate in subsequent experiments. By automating the tedious and time-consuming task of colony picking, it frees up researchers' time for more critical activities and accelerates the pace of scientific discoveries.
Can the ClonePix 2 Mammalian Colony Picker handle different plate formats?
Yes, the ClonePix 2 Mammalian Colony Picker is compatible with various plate formats, including 6-well, 12-well, 24-well, 48-well, and 96-well plates. This flexibility allows researchers to work with different cell densities and enables seamless integration into their existing experimental setups.
How does the ClonePix 2 Mammalian Colony Picker improve the reproducibility of clone selection?
The ClonePix 2 Mammalian Colony Picker eliminates the subjective nature of manual colony picking, which can introduce inconsistencies and errors. By automating the process, it ensures precise and reproducible picking based on predefined criteria. This improves the consistency of clone selection across experiments and enhances the reliability of research outcomes.
Latest Resources
Featured Applications
Customer Breakthrough
SUCCESS STORY


