Together, we're assuring validation of next-generation therapies to accelerate their time to market.
Data integrity is the cornerstone of bringing new therapeutics to market, assuring that every step from research to clinical trials is reliably documented.
Traceable, audit-ready software and hardware provide secure and compliant data management, enabling faster regulatory approvals and improved patient outcomes.
Through validation, we help next-generation therapies reach patients sooner, driving meaningful progress in human health, together.
Insider Insights
From our Insider Insights blog series, experts share both quick reactions and deep reflections on the importance of data integrity for the future of next-generation therapeutics.
Timothy Bolus, MPM, MBA Senior Compliance Program Manager
Tim leverages 25 years of experience in diagnostic labs, manufacturing, clinical development, and the GxP space to bring a customer-focused perspective to the Compliance Portfolio Marketing team.
He collaborates closely with sales and marketing to develop strategies for promoting GxP products and services. Additionally, as a product manager, Tim works with software engineering teams to create innovative software solutions, enhancements, and features.

In the News
Recent announcements showcase partnerships, next-gen technologies, and expert perspectives on industry trends inspiring better science.

Supporting the Science
From close collaborations to independent discoveries, we support scientists as they uncover new insights in complex biology.
Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays
Protocol showing how GxP-compliant software played a role in ensuring the integrity and traceability of data throughout the development and validation of SARS-CoV-2 neutralization assays, supporting regulatory compliance and facilitating the advancement of COVID-19 vaccine research.
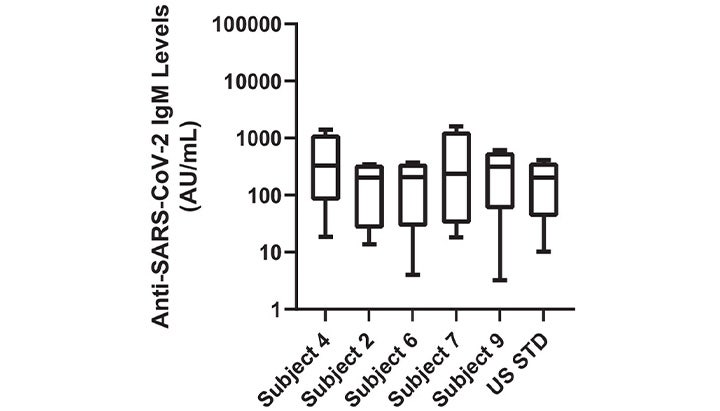
Selection, Characterization, Calibration, and Distribution of the U.S. Serology Standard for Anti-SARS-CoV-2 Antibody Detection
Research article showing how the Abbott Alinity m SARS-CoV-2 assay, operating within a GxP-compliant framework, facilitated rigorous validation of assay performance, ensuring data integrity and regulatory compliance in the evaluation of SARS-CoV-2 diagnostic testing.
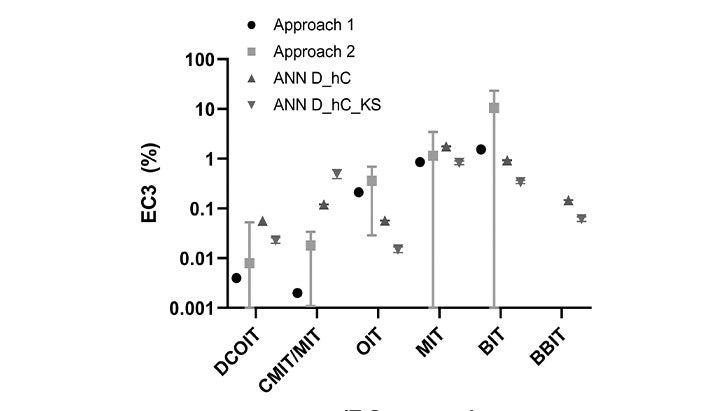
Application of Defined Approaches to Assess Skin Sensitization Potency of Isothiazolinone Compounds
Research article showing how SoftMax Pro GxP software—used to ensure compliance with GLP and GMP standards—analyzed data from assays to assess skin sensitization potential while maintaining data integrity and regulatory compliance.
Catalyzing Breakthroughs with Innovative Technology
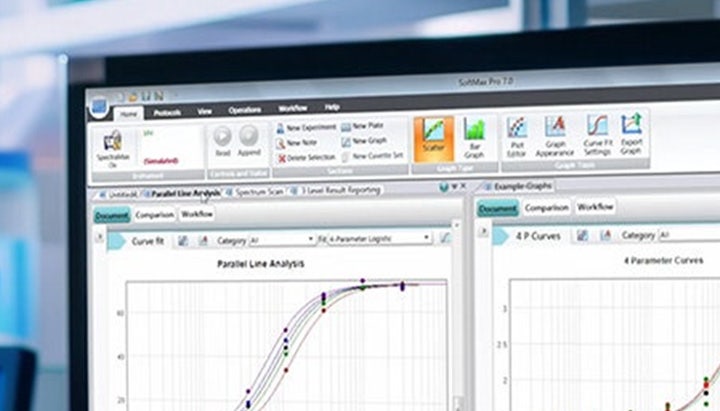
SoftMax Pro GxP Software
For What’s Next: Helping researchers achieve full FDA 21 CFR Part 11 and EudraLex Annex 11 compliance through streamlined workflows in our most secure software.
GxP Compliance Solutions
For What's Next: Proven data integrity solutions from the most cited brand in the industry with over 12,300 citations.

Multi-mode Microplate Readers
For What’s Next: Maintaining 3-day cell viability up to 2x higher in microplate-based assays*



