
Application Note
Cytotoxicity assessment using automated cell imaging and live/dead assays
- Utilize an efficient no-wash homogenous assay protocol to measure cell viability
- Quantify live or dead cells accurately
- Generate statistically relevant results quickly with preconfigured analysis modules
Matthew Hammer | Applications Scientist | Molecular Devices
Oksana Sirenko, PhD | Sr. Applications Scientist | Molecular Devices
Introduction
Live/dead assays are utilized in a wide variety of research applications including investigation of cytotoxic effects of various compounds, treatments, or changes in gene expression. Automated cellular imaging and analysis provides an optimal method to assess cell viability and cell death. Here, we describe the use of the ImageXpress® Pico Automated Cell Imaging System CellReporterXpress® Automated Image Acquisition and Analysis Software to image cells treated with EarlyTox™ Live/Dead Assay Kit
The EarlyTox Live/Dead Assay Kit contains markers for mammalian live and dead cells. Viable cells are stained with an intense green fluorescence in the cytosol by calcein AM. Non-fluorescent Calcein AM permeates the intact cell membrane where the acetoxymethyl (AM) group is cleaved by intracellular esterases, yielding the fluorescent calcein molecule. The dead cell marker, Ethidium homodimer-III (EthD-III), is non-fluorescent and non-permeable to an intact plasma membrane. When cell membrane integrity is compromised in association with cell death, EthD-III enters the cell and binds to nucleic acids, resulting in a bright red fluorescence in dead cells.Cytotoxic events that affect cell membrane integrity can be accurately assessed using this method. The assay kit enables characterization of a full concentration-response profile of test compounds. The no-wash, homogeneous assay eliminates washing steps that can wash away dead and dying cells. Fluorescent signals from calcein and EthD-III can be detected and utilized to produce high quality images and analysis using the ImageXpress Pico system and CellReporterXpress software.
Materials
- EarlyTox Live/Dead Assay Kit
- Explorer Kit (2-plate size, Molecular Devices cat. #R8340)
- Bulk Kit (10-plate size, Molecular Devices cat. #R8341)
- HeLa cells (ATCC P/N CCL-2)
- HeLa media
- Minimum Essential Medium complete media supplemented with glutamine and serum
- Staurosporine (Sigma cat. #S5921)
- Mitomycin C (Sigma cat. #M4287)
- 384-well black, clear-bottom microplates (Corning Falcon cat. #62406-490)
- ImageXpress Pico System and CellReporterXpress software
Methods
HeLa cells, plated at 5,000 cells/well into a black, 384-well clear-bottom microplate, were grown overnight in a 37°C, 5% CO2incubator. The cells were treated for 24 hours with staurosporine (general protein kinase inhibitor and potential anti-cancer therapeutic) or mitomycin C (potent DNA crosslinker and chemotherapeutic) in quadruplicates with a 1:3 serial dilution starting at highest concentrations of 10 μM staurosporine and 300 μM mitomycin C.
After the compound treatment, the cells were stained with the Live/Dead assay kit reagents in combination with Hoechst 33342 nuclear dye (Thermo Fisher Scientific). Half of the volume in each well was removed and replaced with a 2X stain solution of Calcein AM and EthD-III. The final concentrations of stains were 2 μM Calcein AM and 3 μM EthD-III. The plates were then incubated at 37°C, 5% CO2for 30 minutes prior to the addition of Hoechst (6 μM final concentration). The cells were incubated at 37°C, 5% CO2for an additional 15 minutes. Immediately after the final incubation, the plates were imaged on the ImageXpress Pico system using a 10X Plan Fluor objective and the FITC, Texas Red*, and DAPI channels for imaging Calcein AM, EthD-III, and Hoechst dyes, respectively. At this magnification, one field-of-view can capture up to 4000–4500 cells in a single image, yielding statistically relevant results.
* The original data was generated using the TRITC filter, since Texas Red was not previously available. Texas Red is the recommended filter for the Live Dead application.
Image analysis using Cell Scoring module
Images were analyzed using the Cell Scoring analysis module in CellReporterXpress software. The module identifies and differentiates live or dead cells. The Hoechst staining was used to identify total cells, and then cells were scored positive or negative for the specific stains, Calcein AM or EthD-III. Figure 1 shows images of positive and negative controls treated with and without staurosporine and the associated analysis masks indicating positive and negative cells. Separate analyses were performed to define the numbers and percentages of live (Calcein AM-positive) or dead (EthD-III-positive cells) cells.
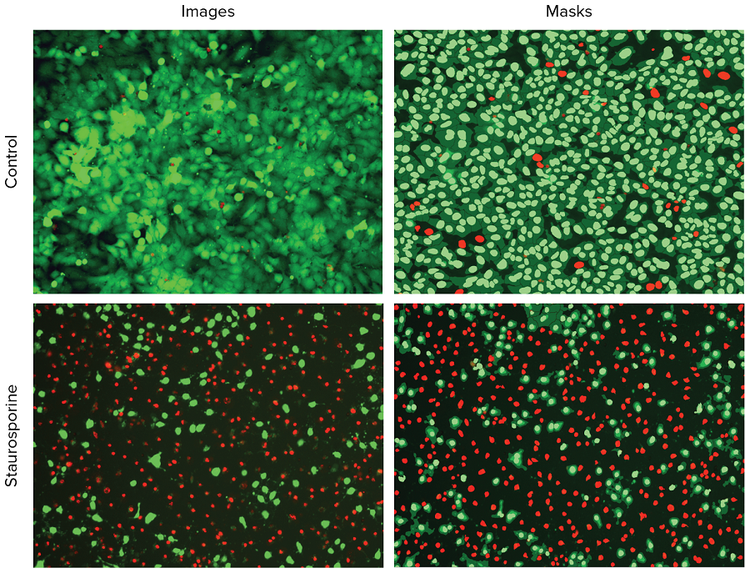
***Figure 1.*Representative images of negative control cells and cells treated with 0.1 μM of staurosporine. Left: 10X images of Hoechst nuclei stained (blue), Calcein AM-stained (green) and EthD-III-stained (red) HeLa cells. Right: Analysis masks show nuclei of live cells in green and nuclei of dead cells in red.
EC50toxicity calculation from dose-response curves
Live and dead cells were imaged, and quantitative cell scoring analysis was performed based on cells staining positive for either Calcein AM (green fluorescence) or EthD-III (red fluorescence) (Figure 1). Treatments of HeLa cells with staurosporine and mitomycin C both showed concentration-dependent increases in percentage of dead cells and decreases in percentage of live cells. Dose response curves displayed in Figure 2 plot the percentage of live cells versus compound concentration with EC50values of 0.569 μM for staurosporine and 223 μM for mitomycin C. The percentage of dead cell curve produced EC50values of 0.492 μM for staurosporine and 6.305 μM for mitomycin C.
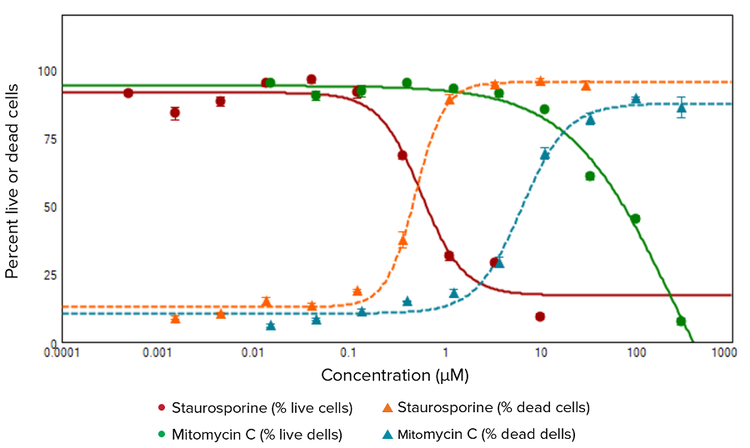
***Figure 2.*Concentration dependencies of the percentages of live and dead cells for HeLa cells treated with different concentrations of staurosporine or mitomycin C. Averages and standard deviations were derived from quadruplicates. The EC50 values produced by these curves are as follows: 0.569 μM staurosporine and 223 μM mitomycin C for % live cells, and 0.492 μM staurosporine and 6.305 μM mitomycin C for % dead cells..
Conclusion
The EarlyTox Live/Dead Assay Kit, in conjunction with the ImageXpress Pico system and CellReporterXpress software, enabled an accurate measurement of live and dead cells with an easy and efficient workflow. The automated imaging and quantitative analysis allows for the testing of cytotoxic compounds and is suited for assessment of cell viability for numerous biological assays.
Matthew Hammer and Oksana Sirenko, PhD | Application Scientist | Molecular Devices
简介
细胞活 / 死测定被广泛应用于各种研究领域, 包括各种化合物的细胞毒性作用、实验处理 或基因表达变化的研究。自动细胞成像和分 析系统提供了一种评价细胞活性和细胞死亡 的最佳方法。在本篇应用文献中,我们介绍 了使用 ImageXpress®Pico 自动细胞成像系 统以及 CellReporterXpress 自动图像采集和 分析软件对 EarlyToxTM Live/Dead 检测试剂 处理的细胞进行成像。
EarlyTox Live/Dead 检测试剂盒包含了针对 哺乳动物活细胞和死细胞的标记物。活细胞 可被在细胞质中能够发出强烈绿色荧光的 Calcein AM 染色。不发荧光的 Calcein AM 可 穿透完整细胞膜且其乙酰羟甲基酯 (AM) 基团 可被细胞内的脂酶裂解,得到可发出荧光的 钙黄绿素分子。死细胞标记物乙啶二聚体-III (EthD-III) 对完整细胞质膜而言既无荧光也无 穿透能力。当与死亡细胞相关的细胞膜完整 性受到损伤时,EthD-III 进入细胞并与细胞核 结合,在细胞中产生明亮的红色荧光。影响 细胞膜完整性的细胞毒性事件可以使用这种 方法准确的评估。
该检测试剂盒可对测试化合物的全浓度响应 特性进行表征。这种免洗、均相实验可减少 因为洗涤步骤导致的死细胞被冲洗掉的情 况。使用 ImageXpress Pico 系统和 CellReporterXpress 软件能准确检测到来自钙黄 绿素和 EthD-III 的荧光信号并获得高质量的 图像及分析结果。
材料
- EarlyTox Live/Dead Assay Kit
- EarlyTox Live/Dead Assay Kit
- Devices P/N R8340)
- Bulk kit (10-Plate Size, Molecular
- Devices P/N R8341)
- HeLa 细胞 (ATCC P/N CCL-2)
- HeLa 培养基
- 补充谷氨酰胺和血清的最低必需培 养基
- Stauroporine (Sigma P/N S5921)
- Mitomycin C (Sigma P/N M4287)
- 384- 孔黑色, 底透微孔板 (Corning Falcon P/N 62406-490)
- ImageXpress Pico 自动细胞成像系统和 CellReporterXpress 软件
方法
HeLa 细胞以 5,000 细胞 / 孔接种于黑色 384 孔底透微孔板中,37°C, 5% CO2 孵育箱内 生长过夜。用十字孢碱 (staurosporine ) ( 通用蛋白激酶抑制剂和潜在的抗癌治疗药 物 ) 或丝裂霉素 C ( 强效DNA交联剂与化疗 药物 ) 处理细胞 24 小时,做 4 个平行,按 1:3 梯度稀释,起始最高浓度为 10 µM 十 字孢碱和 300 µM 丝裂霉素 C。
化合物处理完成后,用 Live/Dead 检测试剂 结合 Hoechst33342 核染料 (Thermo Fisher) 对细胞进行染色。每孔移去一半体积液体 并替换成 2x Calcein AM 和EthD-III 染色 液。染色液终浓度为 2 µM Calcein AM 和 3 µM EthD-III。在添加 Hoechst ( 6 µM 终 浓度 ) 前,孔板在 37°C, 5% CO2 下孵育 30 分钟。细胞再次以 37°C, 5% CO2 孵育 15 分钟。最后一次孵育结束后立即将孔 板在 ImageXpress Pico 系统上用 10x Plan Fluor 物镜进行成像,荧光通道分别为 针对 Calcein AM 的 FITC 通道、针对 EthD-III 的 Texas Red 通道和针对 Hoechst 染料 的 DAPI 通道。在此放大倍数下,一张单 独 的 图 像 一 个 视 野 下 可 以 采 集 多 达 4000-4500 个细胞,足以获得统计学相关 结果。
使用细胞分类模块进行图像分 析
利用 CellReporterXpress 软件中的细胞 分类分析模块对图像进行分析。此模块可 识别和区分活细胞或死细胞。Hoechst 染色用于识别总细胞,而 Calcein AM 或 EthD-III 作为特异性染色用于将细胞分类 为阳性或阴性。图1展示了用十字孢碱处 理和未处理的阳性细胞和阴性对照以及相 关分析所识别的阳性和阴性细胞。分别进 行分析,以确定活细胞或死亡细胞的数量 和百分比。

***图 1:阴性对照细胞和 0.1 µM 十字孢碱处理的细胞的代表性图像。*左侧:Hoechst 核染色 ( 蓝 色 )、Calcein AM 染色 ( 绿色 ) 和 EthD-III 染色 ( 红色 ) 的 HeLa 细胞 10x 采集到的图像。右侧:分 析识别结果展示了绿色的活细胞核和红色的死细胞核
从剂量-应答曲线计算EC50毒性
对活细胞和死细胞成像,并基于 Calcein AM (绿色荧光) 或 EthD-III (红色荧光) 的 细胞阳性染色进行细胞分类定量分析 ( 图 1 )。用十字孢碱和丝裂霉素 C 处理 HeLa 细胞均展示出浓度依赖性的死细胞百分比 的增加和活细胞百分比的下降。图2所呈 现的剂量应答曲线,活细胞百分比对化合 物浓度,其十字孢碱的 EC50 值为 0.569 µM,而丝裂霉素 C 是 223 µM。死细胞百 分比曲线得到的十字孢碱 EC50 值为 0.492 µM 而丝裂霉素 C 为 6.305 µM。

***图2:不同浓度十字孢碱或丝裂霉素 C 处理 HeLa 细胞得到的活细胞和死细胞百分比浓度依赖曲线。*平均值和标准差来自 4 次重复。从曲线中所得EC50值如下:0.569 µM 十字孢碱和 223 µM 丝裂霉 素 C 的 % 活细胞以及 0.492 µM 十字孢碱和 6.305 µM 丝裂霉素 C 的 % 死细胞。
总结
EarlyTox Live/Dead 检测试剂盒结合 ImageXpress Pico 系统和 CellReporterXpress 软件,能够通过简单高效的工 作流程实现对活细胞和死细胞的精确测 定。自动成像和定量分析使得我们能够测 试毒性化合物并非常适合于大量生物学检 测中对细胞活性的评价。