
Application Note
Multiplexed high-content hepatotoxicity assays using iPSC-derived hepatocytes
- Screen for hepatotoxicity in 96- or 384-well plate format easily and rapidly
- Measure multiple hepatotoxicity effects all in one assay
- Tailor analysis to report relevant output using custom modules
Introduction
Drug-induced hepatotoxicity is an important cause for liver injury and acute liver failure. Thus highly predictive assays for safety and efficacy testing are crucial for improving drug development and reducing drug attrition. Human induced pluripotent stem cell(iPSC)-derived hepatocytes, which exhibit typical characteristics and metabolism of mature cells, are ideal for use in high-content screening in early drug development.
Although protocols for performing standard assays on high-content screening systems are well established, image analyses required to evaluate various forms of toxicity can be complex and custom-driven. In this note, we demonstrate the development of multi-parametric hepatotoxicity assays utilizing the ImageXpress®Micro System. Each well or cell yields multiple compound-induced cellular responses, which are then analyzed using custom modules from MetaXpress®Software.
Getting more information from a standard viability assay
Viability dyes such as Calcein AM can be used to address gross compound toxicity in live cells. iCell Hepatocytes (Cellular Dynamics International) were first treated with various compounds for 72 hours then stained. Images of live cells were acquired using a 10X objective on the ImageXpress Micro System, then total nuclei count (Hoechst counterstain) and cytoplasm area of living cells (Calcein AM) were analyzed using the Multi-Wavelength Cell Scoring Application Module of MetaXpress Software(Figure 1).
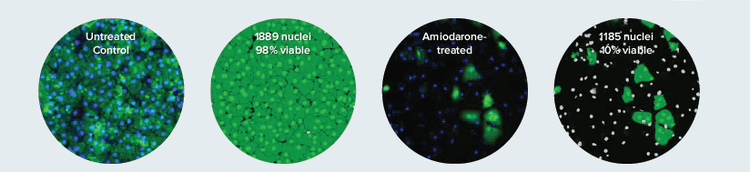
Figure 1. A viability dye yields more accurate toxicity information than nuclear count. Live cells stained with Calcein AM are identified using Multi-Wavelength Cell Scoring Module. The masks on the right show nuclei belonging to live cells in bright green and nuclei in dead cells in grey. Although a similar number of nuclei are counted in control vs. treated wells, it is clear from the percent viable cells that Amiodarone is highly toxic. Additional measurements from the cytoplasm of the live cells (green mask), such as cell area and average intensity can yield insight into the toxic effect mechanism.
Single-dye mitochondrial toxicity assay
Mitochondrial depolarization is an early signal of hypoxic damage or oxidative stress. Mitochondria membrane potential can be assessed by JC-10 dye. In intact mitochondria, orange J-aggregates are visible but, upon membrane depolarization, the dye leaks into the cytoplasm and fluoresces in the fluorescein wavelength. Hepatocytes were treated for 60 minutes with compounds and stained with JC-10 before imaging on the ImageXpress Micro System. A custom module was used to quantitate both the healthy mitochondria which retain the stain and the amount of dye that had leaked into the cytoplasm. The ratio of the two intensities produces a more robust analysis than either measurement alone (Figure 2).
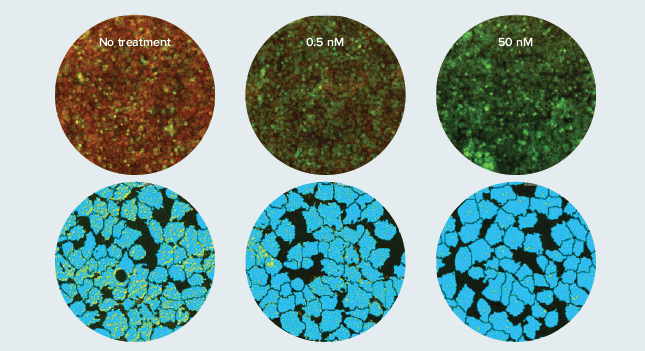
Figure 2. Valinomycin disrupts mitochondria membrane potential. Hepatocytes treated with Valinomycin for 60 minutes. Live cells were stained with JC-10 and imaged with a 10X objective. Top: Overlay of green cytoplasm and red mitochondria. Bottom: Resulting mask (zoomed) after analysis with a custom module shows mitochondria identified (yellow) in a dose response to the compound.
Phospholipidosis and steatosis are common signs of hepatotoxicity
Some drugs may cause phospholipidosis and steatosis, which are lipid metabolism disorders characterized by excess accumulation of phospholipids and neutral lipids in tissues. The amount and distribution of both phospholipids and neutral lipids in liver cells can be detected using imaging methods and quantitated on a per cell basis. Figure 3 depicts an experiment where hepatocytes were plated in 384-well plates and incubated for four days before treatment with selected compounds for 24 hours.
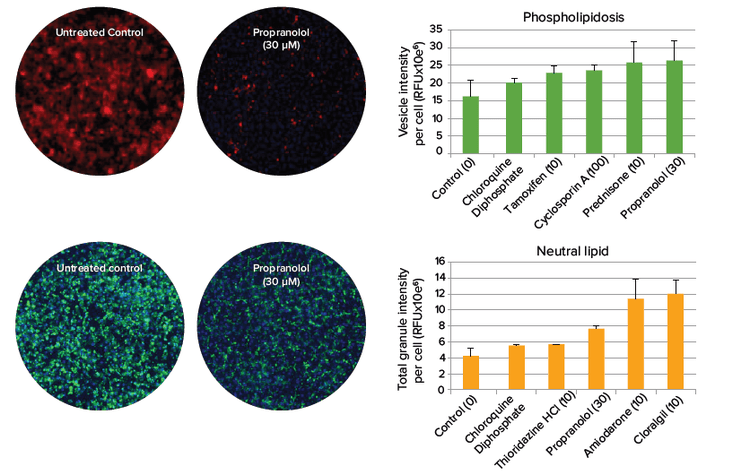
Figure 3. Phospholipidosis and steatosis after compound treatment. Phospholipid (top) and neutral lipid (bottom) staining in hepatocytes imaged with a 10X objective. Cells positive for lipid staining (right) give insight into the specific mechanism of compound toxicity.
More information from your toxicity assays
The Custom Module Editor of MetaXpress Software allows scientists to perform multiplexed assays by specifying custom parameters of interest. Custom modules have been demonstrated that quantitate cell size or shape, total number of viable cells, and apoptosis (Figure 4). These modules can be applied to images and run in MetaXpress®PowerCore™High Content Distributed Image Analysis Software in large batches for high throughput applications.
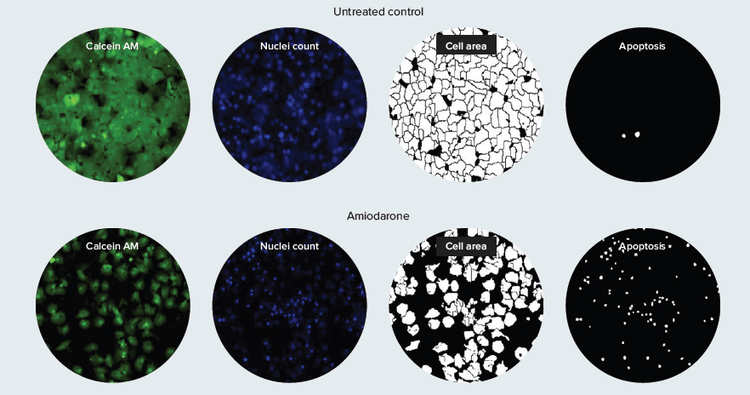
Figure 4. Multi-parametric hepatotoxicity evaluation. This example of a custom module reports measurement of cell area and incidence of apoptosis as well as number of live cells remaining in hepatocytes after compound treatment.
A complete solution to multi-parametric hepatotoxicity screening
Multi-parametric image analysis greatly increases assay sensitivity and provides valuable information about mechanisms of compound toxicity. With the Custom Module Editor in MetaXpress Software, scientists have the freedom to design tailored image analysis modules and rapidly analyze toxicity assays to report relevant output only. Molecular Devices offers flexible high-content screening solutions for assessing hepatotoxicity that go far beyond simple live/dead assays.
导言
药物引发的肝毒性是造成肝损伤和急性肝衰竭的重要原 因之一。因此如何高效的检测药物的有效性和安全性对 于促进药物研发和减少药物消耗至关重要。人诱导多能 干细胞(iPSC)衍生的肝细胞具有典型的成熟细胞的特征 和代谢形式,这对于我们用高内涵筛选药物是非常理想 的。
虽然对于如何利用高内涵进行药物筛选已经有了很多标 准的程序,但是对于如何通过图像分析评估不同毒性却 仍非常复杂且大多依靠经验。本文中我们阐述了如何利 用 ImageXpress® Micro 系统对多参数肝毒性进行分 析。每一个孔或者细胞都会产生多种化合物诱导的细胞 反应,而这些反应可以 用 MetaXpress® 软件的自定义 模块进行分析。
从标准活性检测中获得更多信息
细胞活性染料(例如 Calcein AM)可以用来检测 活细胞中的化合物毒性。首先用各种化合物对肝 细胞进行 72 小时处理并染色。通过 ImageXpress Micro 系统的 10倍物镜可以得到 肝细胞的图像,然后我们用 MetaXpress 软件 的 Multi-Wavelength Cell Scoring 应用模块 对活细胞的整个核区域 (Hoechst 复染色)和细 胞质区域(Calcein AM)进行分析(图 1)。

图 1. 活细胞染色比单独细胞计数提供更准确的毒性信息。Multi-Wavelength Cell Scoring 应用模块对活细胞 Calcein AM 染色进行分 析。右图中绿色蒙板标记出活细胞的核,灰色为死细胞的核。虽然阴性组和处理组的细胞核数相近,但是从活细胞百分比可看出处理药物 Amiodarone 毒性很强。另外,从细胞质的染色中还可分析细胞面积、荧光强度等,研究化合物的毒理机制。
线粒体毒性的单染分析
线粒体去极化是缺氧损伤和氧化应激的早期标志。我 们可以用 JC-10 对线粒体膜电位进行检测,在完整的 线粒体中,橙色的 J-aggregates 肉眼可见, 但是对 于膜去极化来说,这种染料可以渗入到细胞质中并发 出特定的荧光波长。在用 ImageXpress Micro 系统成 像前先用化合物处理肝细胞 60 分钟并用 JC-10 染 色。系统的自定义模块可以对健康线粒体中保存的染 料和渗透到细胞质中的染料分别定量。通过分析这两 类荧光染色信号强度的比率我们可以得到比之前单一 检测更可靠的数据(图 2)。

图 2. Valinomycin 改变线粒体膜电位。肝细胞加 Valinomycin 处理 60 分钟。活细胞被 JC-10 着色并用 10 倍镜拍摄。上:绿色细胞质 和红色线粒体的叠加图。下:经用户自定义分析的结果蒙板图,识别出来的线粒体的数量与化合物浓度相关。
磷脂质病和脂肪变性是最常见的肝细胞 毒性标志
一些药物会引起磷脂质病和脂肪变性,这是由脂肪代 谢紊乱引起的组织中过多的磷脂质和中性脂肪聚集造 成的。我们可以用成像的方法对肝细胞中的磷脂质和 中性脂肪的分布进行检测,这一检测可以精确到单细 胞水平。图 3 我们列举的实验中肝细胞被种在 384 孔 板中,孵育了 4 天,然后用待测化合物处理 24 小 时。

图 3. 化合物处理造成的磷脂质病和脂肪变性。肝细胞的磷脂(上)和中性脂肪( 下)染色,10 倍镜获取图片。化合物处理的细胞脂质染色呈阳 性(右)说明了化合物毒性的特殊机制。
从毒性分析中获得更多信息
MetaXpress 软件中的自定义模块编辑器可以让科学 家们针对自己感兴趣的参数进行复杂的分析。这种自 定义模块已被证明可以有效的定量细胞大小或形状, 活细胞总数以及凋亡情况(图 4)。这些模块也可以应 用在图像处理上,还可以用 MetaXpress®PowerCore™ High Content Distributed Image Analysis Software 对大量样本 进行高通量筛选分析。

图 4. 多参数肝毒性评价。用户自定义模块例子,分析了化合物处理后细胞面积、凋亡发生率和活细胞数。
多参数肝细胞毒性筛选的完整解决方案
多参数图像分析可以显著的提高分析的灵敏性,并且为我们进一步了解化合物毒性发生机制提供更多有价值的信 息。利用 MetaXpress 软件的自定义模块编辑器,科学家们可以自由的设计和调整图像分析模块并迅速的对毒性 进行分析及得到相应结果。Molecular Devices 为评估肝细胞毒性提供了灵活的的高内涵筛选方案,这种方法远 领先于传统的非死即活的简单检测。