DispenCell Single-Cell Dispenser
Compact, automated cell dispenser for fast, easy and gentle single-cell isolation
A simple single-cell dispenser for proof of monoclonality
The DispenCell™ Single-Cell Dispenser is an automated laboratory instrument designed for fast, easy and gentle single-cell isolation. DispenCell integrates seamlessly into your laboratory workflow, with a plug-and-play approach. Flexible and effortless, DispenCell operates equally under sterile conditions in a culture hood, or on a simple benchtop.
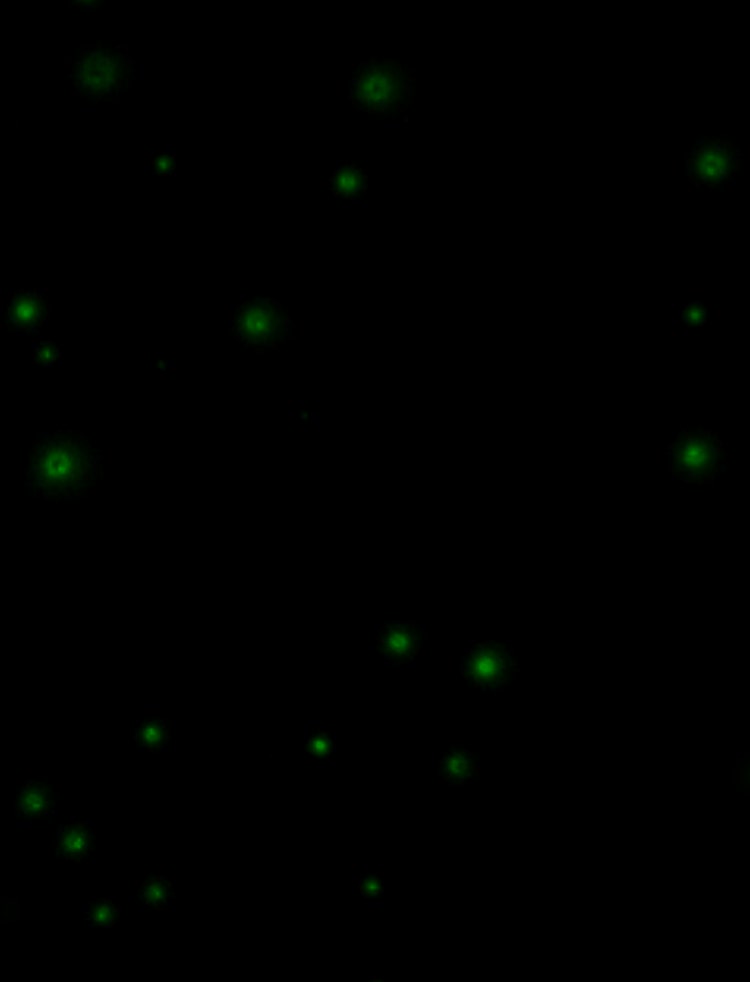
Immediate and traceable proof of clonality
DispenSoft, the single-cell analysis software included with DispenCell, provides immediate and traceable proof of clonality. The instrument is fitted with a sensing tip that detects the passage of cells, allowing users to check for proof of clonality immediately after the cells are dispensed. All dispensing data is automatically stored, allowing for easy generation of a clonality report.
As gentle as manual pipetting
DispenCell has unique technology which allows for extremely gentle handling of the cell sample. Cells are exposed to no more pressure than with manual pipetting (less than 0.2 psi). This allows the instrument to preserve cell viability and outgrowth while simultaneously increasing cell deposition efficiency.
Compact
The small footprint of the DispenCell allows it to fit perfectly into any laboratory setting: under a hood to work under sterile conditions, on a bench top for routine seeding, or in a pre-existing automated workflow.
Features

Proof of clonality
A single-cell analysis software tool provides a traceable proof of clonality report instantly.

Contamination-free
A patented disposable tip ensures clean isolation of single cells and no cross contamination. Certified free from animal products and cytotoxic material.
High cloning efficiency
Unique design ensures gentle dispensing for better viability and cloning efficiency

Easy to use
Easy to set up, and intuitive with a simple interface. No cleaning or calibration required.
Verify monoclonality confidently at day zero with a DispenCell and CloneSelect Imager FL bundle
The manual screening methods traditionally used for cell line development are time-consuming and labor-intensive, creating a great demand for high throughput, automated solutions for such efforts. The general workflow below helps identify the systems that can aid in your research.
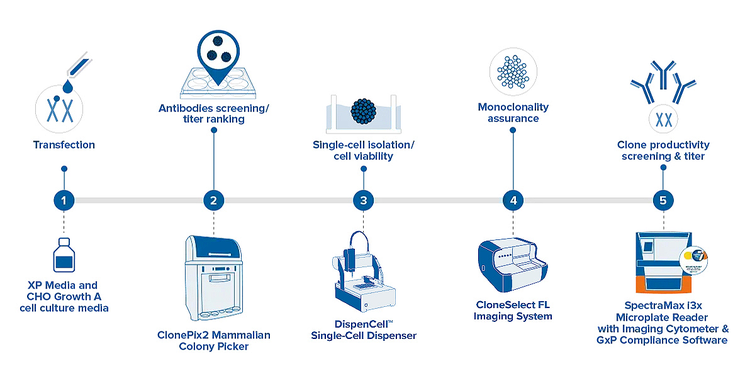
Bundle. Optimize. Save.
The CloneSelect Imager FL
The CloneSelect® Imager FL is a high-throughput automated solution for imaging and analyzing mammalian cells. Tracking the formation of a colony from a single cell is effortless, as barcoded plates are tracked over time. Automated acquisition and analysis provide accurate, objective, and consistent results.
Featuring high-contrast multichannel fluorescent and white light imaging that allows for accurate single-cell detection and proof of monoclonality at day 0, the instrument allows you to streamline your workflow with comparative confluence assays to identify and verify gene edits.
- Demonstrated IND success
- Multichannel imaging and automated confluence
- Rapid single-cell confirmation
FAQ
What is the DispenCell Single-Cell Dispenser?
The DispenCell Single-Cell Dispenser is an innovative instrument designed for precise and efficient dispensing of individual cells into various formats. It enables researchers to isolate and dispense single cells for downstream applications such as clonal expansion, monoclonality assurance, and single-cell genomics.
How does the DispenCell Single-Cell Dispenser work?
The DispenCell Single-Cell Dispenser utilizes advanced microfluidics technology to accurately control the dispensing of individual cells. The tool can effectively detect, size, and dispense particles down to the single-particle resolution based on impedance change that depends on particle volume. It can accommodate a wide range of cell sizes and types, allowing researchers to adjust parameters such as cell concentration and dispensing volume at a gentle pressure of less than 0.2 psi to achieve the desired cell density and distribution.
What are the key features of the DispenCell Single-Cell Dispenser?
The DispenCell Single-Cell Dispenser offers several key features, including high precision and accuracy in single-cell dispensing, adjustable cell concentration, and dispensing volume, compatibility with various plate formats, user-friendly software interface, and the ability to handle a large number of cells in a short amount of time. It also comes with a small footprint that fits in the cell culture hood with gentle pressure dispense for cells and software to trace proof of clonality/monoclonality immediately.
What are the advantages of using the DispenCell Single-Cell Dispenser?
The DispenCell Single-Cell Dispenser provides several advantages, including improved workflow efficiency, reduced cell loss and contamination risks, enhanced reproducibility and consistency in single-cell experiments, increased throughput for high-throughput applications, and the ability to customize cell dispensing parameters to meet specific experimental needs.
What applications can benefit from the DispenCell Single-Cell Dispenser?
The DispenCell Single-Cell Dispenser is ideal for a wide range of applications, including clonal expansion, monoclonality assurance, single-cell genomics, CRISPR screening, antibody discovery, cell line development, rare cell isolation, and the food industry. Its versatility and precision make it a valuable tool for researchers working in various fields of cell biology and biotechnology.
These applications can vary based on the research focus and can be modified to match the desired outcome of the study:
- Monoclonal antibody production – researchers can isolate homogeneous populations of antibody-secreting cells with desired characteristics that could be utilized in therapeutic antibody applications.
- Cell line engineering – allows selection and isolation of cells with specific genetic modifications. Stable cell lines generated using this technique could be valuable tools for gene function, protein production, or drug screening studies.
- Disease modeling – can isolate and establish cell lines that represent specific diseases. Patient-derived cell lines as cellular models for genetic disorders, neurodegenerative diseases, and cancer can be studied. These cells enable several investigative, therapeutic, and personalized medicine approaches.
- High-throughput screening – This can be made feasible by distributing individual cells into multiple multi-well plates and possibly testing large numbers of clones of a specific characteristic simultaneously. This kind of efficiency helps screen large libraries of compounds for toxicity testing, drug screening, and identifying cellular pathways.
- Cell line characterization: screening and characterization of cells for large-scale production by assessing growth rate, viability, stability, and productivity…
- Functional genomics – genomic technologies like single-cell RNA sequencing can be combined with single-cell isolation to study gene expression patterns at the single-cell level. This provides heterogeneity data within cell populations that could help us understand cellular responses and rare cell types further.
What are the specific tasks that the DispenCell Single-Cell Dispenser can perform?
The DispenCell Single-Cell Dispenser can accurately dispense individual cells into various formats, including microplates, microwell arrays, and slides. Incredibly gentle, it preserves the viability of cells, including but not limited to mammalian cells, stem cells, and microbial cells, making it versatile for different research applications.
In what workflows can the DispenCell Single-Cell Dispenser be incorporated?
The DispenCell Single-Cell Dispenser’s precise and efficient dispensing capabilities contribute to the optimization of various workflows by reducing manual labor and enhancing experimental reproducibility. It can be seamlessly integrated into workflows such as single-cell genomics, single-cell cloning, monoclonality assurance, high-throughput screening, and rare cell isolation. Its goal is to isolate and distribute single cells into multi-well plates for downstream applications to perform cell culture, proteomics, genomics, or other functional assays.
How does the DispenCell Single-Cell Dispenser contribute to the optimization of scientific research?
The DispenCell Single-Cell Dispenser optimizes scientific research by providing accurate and controlled dispensing of single cells. This enables researchers to achieve precise cell densities, isolate rare cells for downstream analysis, maintain clonality in cell line development, streamline high-throughput screening, and enhance single-cell genomics experiments for a deeper understanding of cellular heterogeneity.
Can the DispenCell Single-Cell Dispenser be used for CRISPR screening?
Yes, the DispenCell Single-Cell Dispenser is highly suitable for CRISPR screening workflows. It enables the precise dispensing of individual cells, facilitating the isolation of cells with desired genetic modifications induced by CRISPR-Cas9 or other gene editing techniques. This allows for efficient screening and characterization of gene-edited cell populations.
How does the DispenCell Single-Cell Dispenser improve monoclonality assurance?
The DispenCell Single-Cell Dispenser plays a critical role in monoclonality assurance by accurately dispensing single cells. It allows researchers to isolate individual cells and subsequently monitor their growth, ensuring the establishment of clonal cell populations. By minimizing the risk of cell cross-contamination, the DispenCell Single-Cell Dispenser helps to ensure the reliability and reproducibility of experimental results in monoclonality assurance workflows.
Latest Resources
Featured Applications
https://main--moleculardevices--hlxsites.hlx.page/fragments/applications/cell-line-development-2
https://main--moleculardevices--hlxsites.hlx.page/fragments/applications/monoclonal-antibodies-mabs
https://main--moleculardevices--hlxsites.hlx.page/fragments/applications/monoclonality-assurance
https://main--moleculardevices--hlxsites.hlx.page/fragments/applications/single-cell-genomics
https://main--moleculardevices--hlxsites.hlx.page/fragments/applications/single-cell-sorting
How can we help advance your next big discovery?
Our highly-qualified teams are on the frontlines with our customers, conducting remote or on-site product demonstrations, webinars, and more to help you solve your tough research challenges. How can we help you today?
I’d like to…
Applications of DispenCell Single-Cell Dispenser
Specifications & Options of DispenCell Single-Cell Dispenser
** Monoclonal reliability error definition:
Impedance trace reliability error = P(obs > 1|z = 1) = P(obs > 1nz = 1)/P(z = 1)
The monoclonal reliability error is defined as the probability of observing more than one particle/cell when the impedance time trace exhibits one event. The monoclonal reliability is defined as the ones' complement of its error.