
Application Note
Streamline BCA-based protein quantitation on the SpectraMax iD5 reader
- Quantitate proteins rapidly with a 5-minute, room-temperature incubation
- Measure assay absorbance values quickly with minimal background noise on the SpectraMax iD5 reader
- Graph standard curves and calculate sample protein concentrations easily with a preconfigured protocol in SoftMax Pro Software
Introduction
Carole Crittenden | Applications Scientist | Molecular Devices
Cathy Olsen, PhD | Sr. Applications Scientist | Molecular Devices
Marissa Statner | Intern | Molecular Devices
The BCA assay is a two-step colorimetric assay used to quantitate the total protein in a sample. This assay uses the Biuret reaction, in which Cu2+ is reduced to Cu1+ by protein in an alkaline medium. The amino acid backbone forms a color-chelate complex with the copper molecules, allowing for a highly sensitive and selective detection of the cuprous cation. The resulting water-soluble complex exhibits strong absorbance which can be measured on a microplate reader. The original Pierce™ BCA Protein Assay Kit requires incubation for 30 minutes at 37°C and produces a color change that is measured at 562 nm. A newer version of the assay, the Pierce Rapid Gold BCA Protein Assay Kit, simply requires incubation for five minutes at room temperature and yields an intense orange-gold color that is measured at 480 nm.
The SpectraMax® iD5 Multi-Mode Microplate Reader includes an absorbance detection mode optimal for protein quantitation assays. Here, we illustrate how the SpectraMax iD5 reader, in combination with SoftMax® Pro Software, is used to quantitate a cellular protein sample with two different kits, Pierce BCA Protein Assay Kit and Pierce Rapid Gold BCA Protein Assay Kit. The kits include bovine serum albumin, which is used to set up a standard curve from which the concentrations of samples are interpolated and reported automatically by a preconfigured protocol in SoftMax Pro Software. The 5-minute incubation at room temperature used for the Rapid Gold BCA assay, compared to the 30-minute incubation at 37°C required for the original kit, offers time savings and convenience.
Materials
- SpectraMax iD5 Multi-Mode Microplate Reader (Molecular Devices cat. #ID5-STD)
- Pierce BCA Protein Assay Kit (ThermoFisher Scientific cat. #23225)
- Pierce Rapid Gold BCA Protein Assay Kit (ThermoFisher Scientific cat. #A53225)
- RIPA Buffer (ThermoFisher Scientific cat. #89900)
- Halt Protease Inhibitor Single-Use Cocktail (ThermoFisher Scientific cat. #78430)
- 96-well, clear, flat-bottom polystyrene microplate (Greiner Bio-One cat. #655101)
- 293 [HEK-293] cells (ATCC cat. #CRL-1573)
Methods
HEK-293 cells were cultured in a T75 culture flask until sub-confluent. Cells were then washed with 10 mLs of cold phosphate-buffered saline containing calcium and magnesium (PBS). 2 mLs of cold RIPA buffer were added to the flask and remained on the cells for five minutes, then lysed cells were pipetted up and down several times to release any remaining cellular material from the flask surface. The lysate was divided into two 1.5-mL microcentrifuge tubes, and Halt Protease Inhibitor was added. Lysates were centrifuged at 15,000 rpm for 20 minutes, and supernatant was used to make a 3-fold dilution series of the unknown sample in PBS. A dilution series was performed to ensure that some sample dilutions would fall within the assay's measurable range.
BSA standard curves were prepared by following the method given in each kit’s user guide. 12 mL of each BCA working reagent were prepared using a 50:1 ratio of Reagent A to Reagent B. 25 µL of protein standards or cell sample were transferred in triplicate (one set per assay) to clear 96-well microplates. One plate received 200 µL of the BCA working reagent, and the other received 200 µL of the Rapid Gold BCA working reagent. The BCA assay plate was incubated for 30 minutes at 37°C, and the BCA Rapid Gold assay plate was incubated for five minutes at room temperature.
Absorbance values were read on the SpectraMax iD5 reader using a preconfigured BCA assay protocol (the SpectraMax iD3 reader is also suitable for these assays, as it has the same absorbance performance as the SpectraMax iD5 reader). The BCA assay plate was read at 562 nm, the original wavelength setting in the protocol, while for the BCA Rapid Gold assay the wavelength setting was changed to 480 nm prior to reading the plate.
BSA standard curves were generated by SoftMax Pro Software, and a quadratic curve fit was applied, as recommended in the assay kit user guides. The standard curve for each assay was used to calculate the concentrations of the HEK-293 cell samples. Refer to Figure 1 for a visual representation of the workflow for each kit.
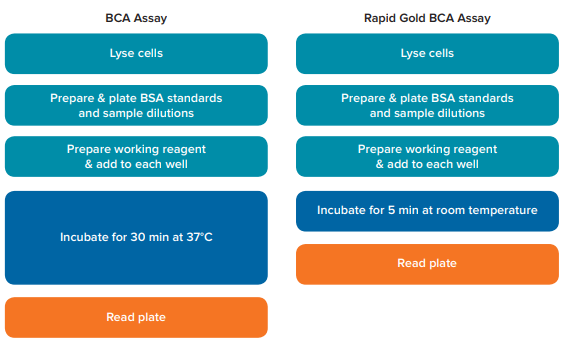
Figure 1. Comparison of assay workflow. Steps in the experimental procedures for the BCA assay and Rapid Gold BCA assay are depicted. The BCA assay requires an incubation of 30 minutes at 37°C, while the Rapid Gold BCA assay only requires a 5-minute incubation at room temperature.
Results
The BSA standard curves for the BCA assay and Rapid Gold BCA assay both yielded r2 values above 0.99 using a quadratic curve fit (Figure 2). The range of OD values seen from low to high BSA standard was larger for the BCA Rapid Gold assay than for the BCA assay.

Figure 2. BSA standard curves. Standard curves were plotted using the quadratic curve fit in SoftMax Pro Software for the BCA (green) and BCA Rapid Gold (blue) assays. For BCA, r2 = 0.997, and for BCA Rapid Gold, r2 = 0.999. For each curve, n=3.
Cell lysate concentrations were interpolated from the standard curves, calculated automatically by SoftMax Pro Software, and displayed in a group table (BCA Rapid Gold group table is shown in Table 1). For the BCA Rapid Gold assay, the original cell lysate sample concentration was found to be 680 µg/mL while for the BCA assay it was about 640 µg/mL, thus similar concentrations were calculated despite the difference in OD ranges observed between the two standard curves.
B4
B5
B6
0.838
1.166
0.698
386
554
317
C4
C5
C6
0.486
0.529
0.388
217
237
172
D4
D5
D6
0.192
0.179
0.151
84
78
66
E4
E5
E6
0.056
0.066
0.051
R
R
24
29
22
Table 1. Example of a group table for the BCA Rapid Gold assay. For each OD value measured, a concentration was interpolated from the standard curve. OD values falling outside the range covered by the standard were flagged as ‘R’. For each mean concentration, an adjusted concentration was calculated by multiplying the mean concentration by the dilution factor of the sample. Samples 02, 03, and 04 gave consistent adjusted concentrations, so the original sample’s concentration was estimated to be 680 µg/mL.
Conclusion
The BCA Rapid Gold assay enables microplate incubation at room temperature for a total of five minutes, compared to the original BCA assay, which requires the microplate to be incubated at 37°C for 30 minutes. Both assays yielded standard curves with excellent r2 values and similar calculated sample concentrations.
The SpectraMax iD5 reader rapidly performs absorbance measurements that are used to plot standard curves and calculate sample concentrations using a preconfigured protocol in SoftMax Pro Software. The protocol contains settings for reading the plate, as well as template groups that allow a user to easily set up analysis, including calculation of sample concentrations from a standard curve.
简介
Carole Crittenden | Applications Scientist | Molecular Devices
Cathy Olsen, PhD | Sr. Applications Scientist | Molecular Devices
Marissa Statner | Intern | Molecular Devices
BCA 法是一种通过比色分析来对蛋白质进 行定量的方法。该方法利用蛋白在碱性环 境中将 Cu2+ 还原成 Cu+ ,BCA 与 Cu+ 形成 有颜色的络合物。该水溶液络合物在特定 波长下有强烈的吸光值,且在一定范围内 蛋白浓度与光吸收值成正比,这就使得我 们可以利用微孔板读板机进行高通量、高 灵敏度的蛋白定量检测。早期的 PierceTM BCA 蛋白检测试剂盒需要 37℃ 孵育 30 分 钟,在 562 nm 处检测光吸收变化。新版 Pierce Rapid Gold BCA 蛋白检测试剂盒仅 需室温孵育 5 分钟就会产生强烈的橙-金络 合物,在 480 nm 处检测。
SpectraMax iD5 多功能微孔板读板机具 有光吸收检测功能,可以进行蛋白定量 检测。下面,我们介绍一下如何用 SpectraMax iD5 配套 SoftMax®Pro 软件, 用 Pierce BCA 蛋白检测试剂盒和 Pierce Rapid Gold BCA 蛋白检测试剂盒进行检 测。以上试剂盒均包括标准品牛血清白蛋 白,SoftMax Pro 预设的模板可以根据标 准曲线将待测蛋白的浓度自动计算出来。 Pierce Rapid Gold BCA 蛋白检测试剂盒 仅需室温孵育 5 分钟相比 Pierce BCA 试剂 盒 37℃ 孵育 30 分钟,可节省大量时间且 提高操作简便性。
材料
- SpectraMax iD5 多功能微孔板读板机 (Molecular Devices cat.#ID5-STD)
- Pierce BCA 蛋白检测试剂盒 (ThermoFisher Scientific cat.#23225)
- Pierce Rapid Gold BCA 蛋白检测试剂盒 (ThermoFisher Scientific cat.#A53225)
- RIPA 缓冲液 (ThermoFisher Scientific cat.#89900)
- 蛋白抑制剂混合物 (ThermoFisher Scientific cat.#78430)
- 96 孔透明平底聚苯乙烯微孔板 (Greiner Bio-One cat.#655101)
- 293 [HEK-293] 细胞 (ATCC cat.#CRL -1573)
方法
HEK-293 细胞在 T 75 细胞培养瓶中培养。 先用 10 mL 磷酸盐缓冲液 (PBS) 冲洗细胞, 加入 2 mL RIPA 缓冲液孵育 5 分钟,枪头 上下吹打数次使得瓶壁上的细胞也消化下 来。将消化液均分至 2 个 1.5 mL 离心管中, 加入蛋白抑制剂。消化液 15000 rpm 离心 20 分钟,收集上清作为待测样本,以 3 倍梯 度稀释待测样本。梯度稀释是为了待测样 本浓度在标准曲线检测范围内。
BSA 标准品根据各自试剂盒中的操作说明 准备。试剂 A 和试剂 B 按照 50∶1 配置,得 到 12 mL BCA 工作液。将 25 µL 的蛋白标 准品和待测细胞样本分别加入 96 孔透明微 孔板中,每个样本 3 个重复。其中一个微孔 板加入 200 µL BCA 工作液,另一个加入 200 µL Rapid Gold BCA 工作液。BCA 检测试剂 板 37℃ 孵育 30 分钟,Rapid Gold BCA 检测 试剂板室温孵育 5 分钟。
用 SpectraMax iD5 多功能微孔板读板机读 取光吸收值,采用预设的 BCA 分析程序 ( SpectraMax iD3 多功能微孔板读板机与 SpectraMax iD5 有同样优异的光吸收检测 表现,同样适用该类检测 ) 进行检测。BCA 检测板在 562 nm 检测光吸收值,预设程序 中检测波长就是 562 nm,无需做任何更改 即可直接读板;BCA Rapid Gold 检测板在 读板前需要更改检测波长至 480 nm。
SoftMax Pro 软件已根据检测试剂盒说明书 预设了标准曲线,读板后会自动拟合出一 条 BSA 标准曲线。每次分析都可以用标准 曲线来计算 HEK-293 细胞样本中的蛋白浓 度。图 1 是两个试剂盒的工作流程。

图 1 比较两种检测试剂盒的工作流程。 上图是 BCA 检测试剂盒和 Rapid Gold BCA 检测试剂盒的实验 流程图。BCA 检测试剂盒需要 37℃ 孵育 30 分钟,而 Rapid Gold BCA 检测试剂盒仅需室温孵育 5 分钟
结果
BCA 和 Rapid Gold BCA 检测试剂盒的 BSA 标准曲线采用二次曲线拟合 r2 值都大于 0.99 ( 图 2 )。Rapid Gold BCA 检测试剂盒的 BSA 标准品无论低浓度还是高浓度的 OD 值都大 于 BCA 检测试剂盒的。

F图 2 BSA 标准曲线。 标准曲线在 SoftMax Pro 软件中采用二次曲线拟合,其中 BCA 检测试剂盒为绿 色,Rapid Gold BCA 为蓝色。BCA 检测试剂盒的 r2 = 0.997,Rapid Gold BCA r2 = 0.999。每个样本 有 3 个复孔,n = 3
在 SoftMax Pro 软件中,未知样品蛋白浓 度会自动带入标准曲线中计算出来,并以 表格形式在软件中显示 ( 表 1 是 Rapid Gold BCA 检测试剂盒的待测样本浓度 )。
B4
B5
B6
0.838
1.166
0.698
386
554
317
C4
C5
C6
0.486
0.529
0.388
217
237
172
D4
D5
D6
0.192
0.179
0.151
84
78
66
E4
E5
E6
0.056
0.066
0.051
R
R
24
29
22
表1 一组 Rapid Gold BCA 检测数据。 每个检测出的 OD 值,都会自动带入标准曲线计算浓度。如 果检测出的 OD 值超出了标准曲线的范围,会用“R”标出。每个样品的平均浓度都会对应一个校 正浓度,校正浓度是根据每个样品的稀释倍数计算出的未稀释样品的浓度值。样本 02、03 和 04 的 校正后浓度基本一致,估算出原始样本的蛋白浓度为 680 µg/mL
结论
Rapid Gold BCA 检测试剂盒仅需室温孵育 5 分钟,与原来的 BCA 检测试剂盒 37℃ 孵 育 30 分钟相比,可节省大量时间,提高 工作效率。两种检测试剂盒的标准曲线都 得出了出色的 r2 和相近的待测样品浓度。
SpectraMax iD5 多功能微孔板读板机能够 快速、精准的进行光吸收检测,SoftMax Pro 软件中的预设程序能够直接给出标准曲 线和未知样品浓度。预设程序包括读板设 置和样品分组信息等设置,因此未知样品 浓度可直接根据标准曲线计算出来,并在 软件中显示。