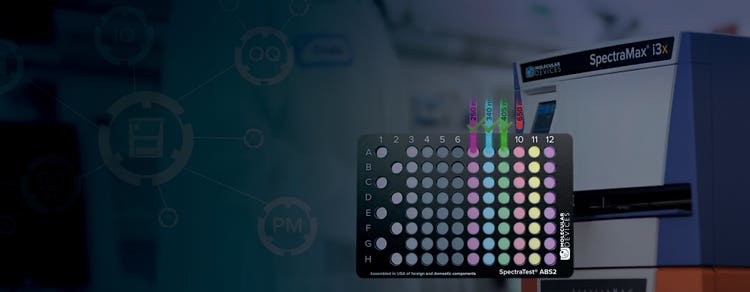
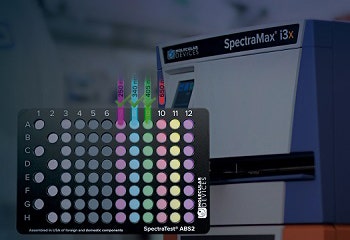
SpectraTest Validation Plates and Recertification
Microplate reader qualification and plate recertification
SpectraTest Validation Plates and Recertification
The SpectraTest® Validation Plates are valuable tools for verifying that absorbance-, fluorescence-, and luminescence-capable microplate readers are operating correctly for GMP and GLP laboratories.
Annual recertification of your validation plates ensure that they meet specifications and give you confidence in the performance of your instrument.

Measure absorbance, fluorescence, and luminescence with confidence
Use photometric accuracy tests to verify how close an absorbance measurement is to the true value, which is traceable to the National Institute of Standards and Technology (NIST) and NMI (National Metrology Institutes).
Determine the accuracy and repeatability of the wavelength selection with the monochromator, which includes separate tests for excitation and emission.
Qualify the kinetic noise that measures the stability of the optical system at low and high signals, including separate tests for spikes and drift at low and high levels.

Ensure data accuracy of your reader
We have the necessary knowledge and equipment to recertify SpectraTest Validation Plates
Features

SpectraTest FL1 Fluorescence Validation Plate
The SpectraTest FL1 plate enables PMT matching to ensure linearity of signal over a greater dynamic range, and RFU (Relative Fluorescence Unit) scaling for optional adjustment of the RFU output.
Data Analysis
Preconfigured protocols in SoftMax® Pro GxP Software automatically generate and analyze data. In addition, compliance tools for FDA 21 CFR Part 11 and EudraLex Annex 11 allow for controlled access to test results with audit trails.

SpectraTest ABS2 Absorbance Validation Plate
This next generation SpectraTest ABS2 plate measures the sensitivity of the optical system ensuring that it is uncompromised by stray light. The plate includes automated certificate value entry and is compatible with our StakMax® Microplate Handling System.

Recertification service
- Molecular Devices is ISO 17025 accredited† by American Association of Laboratory Accreditation (A2LA), which requires rigorous outside auditing on a regular basis so that you can be assured of the accuracy of your certificate with respect to NIST and NMI traceable standards
- Gold Standard and Reference Standard Molecular Devices microplate readers are used for recertification so that your certificate values are traceable
- If validation plate repair is needed, only factory-approved parts that meet our internal specifications for these plates are used
- Rapid turnaround time of five business days or less; expedited service is available as needed

SpectraTest LM1 Luminescence Validation Plate
The SpectraTest LM1 plate enables tests for background noise and spikes, crosstalk to measure light from adjacent wells, and checks for left-to-right bias from the left to the right edge of the plate.
Recertification Service

Annual recertification of your validation plates ensure that they meet specifications and give you confidence in the performance of your instrument. ABS2, FL1, and LM1 Validation Plates sent to Molecular Devices for recertification at our ISO 17025 accredited lab are disassembled, cleaned, calibrated, an then returned with a new certificate of calibration.
- Ensure data accuracy of your reader
- Monitor reader performance on demand to ensure data quality is not compromised between PM/OQ maintenance services
- Minimize downtime by quickly being able to distinguish between reader and assay issues
- Maintain conformance to your validation protocols
Only Molecular Devices has the necessary knowledge and equipment to recertify SpectraTest Validation Plates.
Latest Resources
Customer Breakthrough
Resources of SpectraTest Validation Plates and Recertification
SpectraTest Validation Plates
SpectraMax iD3, iD5, i3x, i3, M2, M2e, M3, M4, M5, M5e, Plus 384, ABS, ABS Plus, FlexStation 3
Requires SoftMax Pro Software version 6.5.1 or higher
Please call
technical support
*Specific read modes or cartridges.