
A Brief History of Patch-Clamping — Part 2: Building the Instrumentation
By a fellow electrophysiologist, for electrophysiologists
By the close of the 19th Century, the field of electrophysiology had a firm grasp on the existence of bioelectricity and the ionic nature of action potentials. However, recording techniques remain crude to today’s standards. We needed better resolution, tighter control, and excellent stability to study membrane dynamics in greater detail. Enter the 20th Century: the age of the instrument.
From Giant Squid Axons to Voltage Clamps: The First Direct Intracellular Recordings
Shortly after, Alan Hodgkin and Andrew Huxley, along with Kenneth Cole and Howard Curtis, inserted intracellular electrodes into the squid axon, enabling direct measurement of membrane potential and action potentials. These recordings made electrophysiology a quantitative science.
Then came the voltage clamp, developed independently by Cole and George Marmont in 1949. This breakthrough allowed scientists to hold the membrane potential constant while measuring ionic currents. For the first time, researchers were able to resolve current-voltage relationships and channel kinetics with temporal precision.
Refining the Model: Ion Selectivity and the Birth of Quantitative Electrophysiology

In the 1940s and early 1950s, the Goldman-Hodgkin-Katz (GHK) equation provided a mathematical framework for membrane potential based on ion permeability. This generalized the Nernst equation to account for multiple ions across membranes, allowing more accurate modeling of actual cells.
In 1952, Hodgkin and Huxley took things even further. Using voltage-clamp data, they mathematically described the gating behavior of Na⁺ and K⁺ channels during an action potential.
Their model remains the gold standard in membrane biophysics, and they (and John Eccles) earned the Nobel Prize in 1963.
Micropipettes and Microelectrodes: The Evolution of Recording Tools
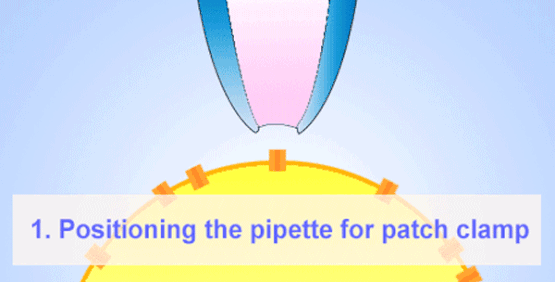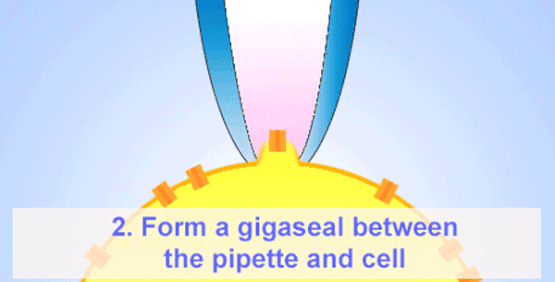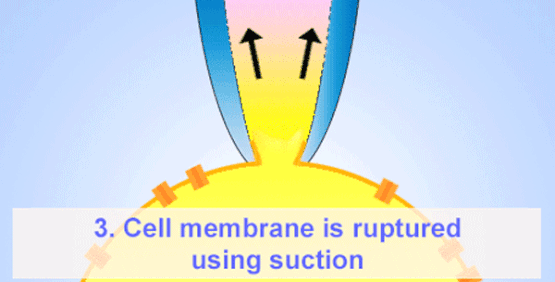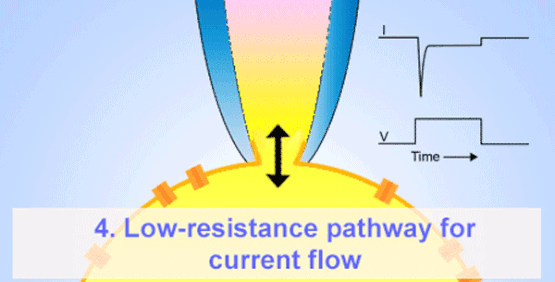
By the late 1960s, Erwin Neher and Hans Dieter Lux introduced fire-polished micropipettes with improved shunt resistance.
They formed tighter seals with the cell membrane using gentle suction, minimizing leaks and noise—crucial for accurate recordings in small cells.
From Intracellular Perfusion to Single-Channel Currents
Throughout the 1970s, intracellular perfusion became a key technique for controlling internal ion composition, pioneered by Peter Baker, Trevor Shaw, and Alan Hodgkin. In parallel, others like Oleg Krishtal, Volodymyr Pidoplichko, and Kai S. Lee developed refined systems using chamber and pipette-based perfusion methods to target single cells with unprecedented control.
However, the most transformative innovation came in 1976 when Neher and Bert Sakmann developed a method to measure single ion channel currents.
By shrinking the tip of the micropipette down to just 1–2 μm and creating a high-resistance (giga-ohm; GΩ) seal with the cell membrane, they achieved the noise isolation necessary to record currents through individual channels—leading directly to the modern patch-clamp technique.
Their landmark 1981 paper, co-authored with Sigworth, Marty, and Hamill, established patch-clamp recording as the gold standard for ion channel analysis. In 1991, Neher and Sakmann were awarded the Nobel Prize in Physiology or Medicine for this work.
Electrophysiology Goes High-Throughput: Automation and Optogenetics
By the early 2000s, the need for scale in drug discovery and functional genomics pushed electrophysiology toward automation. In 2002, Axon Instruments (founded by Alan Finkel) introduced the PatchXpress 7000A, the first automated multichannel patch-clamp system. This leap transformed patch-clamping from a painstaking one-cell-at-a-time method to a viable screening tool for ion channel pharmacology.
Meanwhile, a revolution was brewing on another front. In 2004, Karl Deisseroth, Ed Boyden, and Zhuo-Hua Pan developed optogenetics, enabling light-driven control of ion channels.

Electrophysiologists could now manipulate and record neural activity with unprecedented temporal and spatial precision—merging optics with electrophysiology in a way few had imagined.
Where We Stand Today
Patch-clamp remains one of the most potent tools in cellular neuroscience, cardiac physiology, and ion channel research. The technique has evolved tremendously from manually guided pipettes to automated, multi-well systems—but its foundations lie in the meticulous work of generations past.
In the next installment, we will explore how scientists use patch-clamp electrophysiology today in cutting-edge neuroscience, drug development, and beyond—and how this field may evolve in the era of AI, optogenetics, and high-throughput biology.
Want to dive deeper into techniques, tips, and instrumentation?
Download the Axon Guide to Electrophysiology.
References
- Verkhratsky, A., Krishtal, O. A., & Petersen, O. H. From Galvani to patch clamp: the development of electrophysiology. Pflugers Arch. 453, 233–247 (2006).
- Seyfarth, E.-A. Julius Bernstein (1839–1917): pioneer neurobiologist and biophysicist. Biol. Cybern. 94, 2–8 (2005).
- Pearce, J. Emil Heinrich Du Bois-Reymond (1818–96). J. Neurol. Neurosurg. Psychiatry 71, 620 (2001).
- Verkhratsky, A., & Parpura, V. History of electrophysiology and the patch clamp. Methods Mol. Biol. 1183, 1–19 (2014).