
Exploring the power of SoftMax Pro GxP Software - A four-part tutorial webinar series
Bonus: Download free data and protocol files
Meet FDA guidelines in GMP/GLP laboratories—from setting up and analyzing an ELISA assay to more advanced techniques such as parallel line analysis
We invite you to participate in our four-part tutorial webinar series focused on the comprehensive functionality of SoftMax Pro GxP Acquisition and Analysis Software. Attending these webinars will give you a thorough understanding of the software's features and capabilities, enabling you to optimize your data analysis, reporting, and compliance processes.
Our team of experts will guide you through practical examples and real-life applications, demonstrating how SoftMax Pro GxP software can enhance your workflow and streamline your scientific endeavors. From data analysis techniques to ensuring regulatory compliance, this series will cover a wide range of topics essential for efficient and effective utilization of SoftMax Pro GxP software.
SoftMax Pro GxP Software Tutorials:
Part 1 - How to set up an ELISA assay and perform basic analysis
Part 2 - Performing advanced statistical analyses
Part 3 - How to perform a Parallel Line Analysis (PLA)
Part 4 - Helping GMP/GLP labs meet data integrity guidelines: Introducing SQL & protocol workflow in SoftMax Pro 7.1.1 GxP
Register for our comprehensive SoftMax Pro GxP webinar series
Join our four-part tutorial webinar series to unlock the full potential of SoftMax Pro GxP Acquisition and Analysis Software. Gain insights from practical examples and real-life applications, optimizing your data analysis, reporting, and compliance processes.
Unlock your lab's potential: Getting started with SoftMax Pro GxP Software Acquisition and Analysis Software
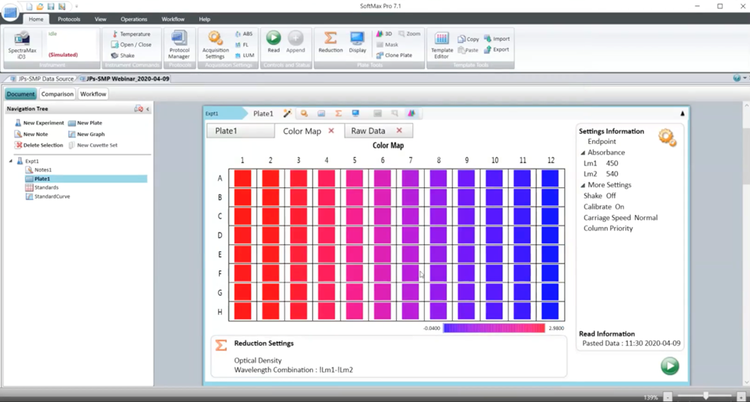
Part 1 - How to set up an ELISA assay and perform basic analysis using SoftMax Pro GxP Software
Gain insights from a live demonstration using the SpectraMax® iD3 Multi-Mode Microplate Reader, software settings, data acquisition, and the basics of analysis, including data reduction, template creation, and standard curve generation using an actual data set.
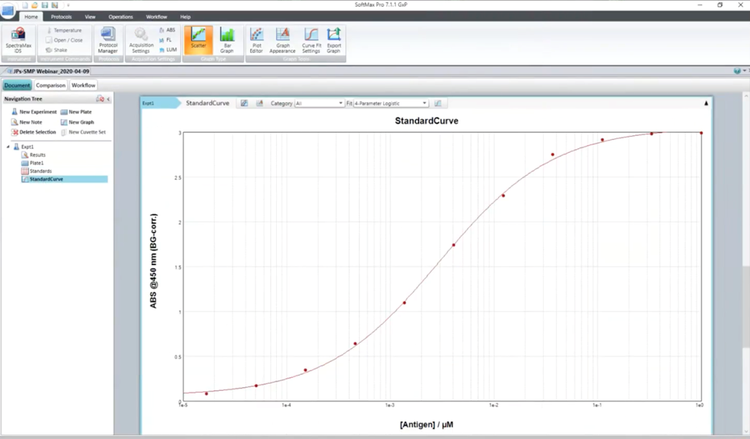
Part 2 - Performing advanced statistical analyses in SoftMaxPro GxP Software
The demonstration includes data comparison with specified criteria using formulas, validation of Pass/Fail criteria, reading unknowns from a standard curve, and flagging wells for drug screening.
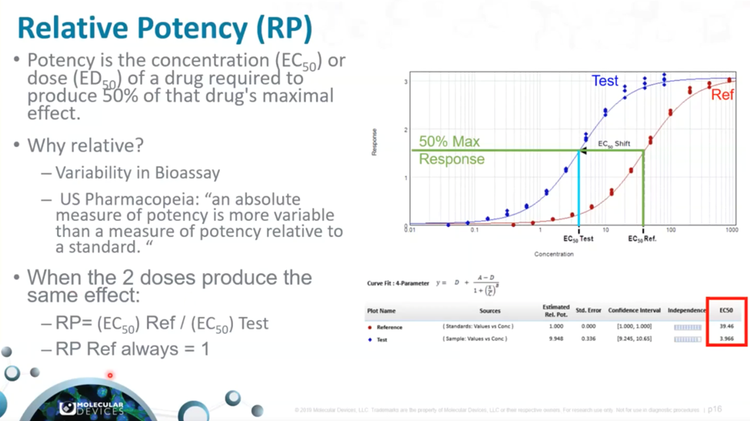
Part 3 - How to perform a Parallel Line Analysis (PLA) in SoftMax Pro Software
Gain hands-on experience as we showcase the practical application of PLA, including data weighting, linear and non-linear curve fitting, and confidence intervals. Unlock the potential of PLA to enhance data accuracy and estimation.
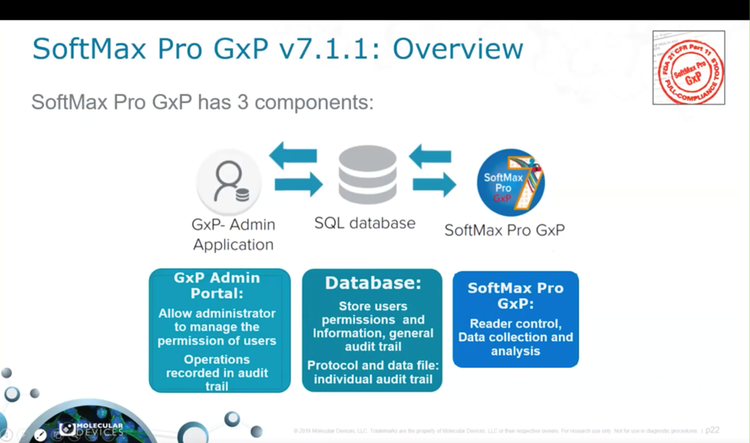
Part 4 - Helping GMP/GLP labs meet data integrity guidelines: Introducing SQL & protocol workflow in SoftMax Pro 7.1.1 GxP
Explore the new features, including a demonstration of the SoftMax Pro 7.1.1 GxP workflow, the system audit trail, and the benefits of the Microsoft SQL Express database structure, ensuring improved data integrity and control over document workflows.
