
Application Note
Determine total aflatoxin in cannabis using an ELISA method
- Save time and money with a high-throughput, low-cost ELISA
- Analyze multiple aflatoxins from a single extract
- Evaluate results rapidly with SoftMax Pro Software
Joyce Itatani | Applications Scientist | Molecular Devices
Cathy Olsen | Sr. Applications Scientist | Molecular Devices
Introduction
As cannabis is legalized in more states across the US, state agencies are setting their own regulations and requirements for growers, retailers, and testing laboratories. For example, in January 2019, cannabis regulations took effect in California, requiring that under state law all cannabis goods must be tested to ensure consumer safety. Potential contaminants of cannabis include mycotoxins, toxic byproducts of fungi of the genus Aspergillus that can cause disease and death in humans or animals when consumed. California’s Bureau of Cannabis Control (BCC) requires testing of cannabis products for the most common and dangerous mycotoxins: aflatoxins B1, B2, G1, G2, and ochratoxin A. The limit set for these toxins is 20 ppb, the same level set by the FDA for most foods. Other states have their own requirements for aflatoxin levels.
Strip tests and enzyme-linked immunosorbent assays (ELISAs) are two common, relatively low-cost methods for mycotoxin testing of food and feed samples. An ELISA provides the advantage of higher throughput, as it is run in a 96-well microplate format, while only two samples at a time can be run on a test strip. AgraQuant Total Aflatoxin 4/40 ELISA kit from Romer Labs can be used to quantify aflatoxin in food and feed components, with the ability to run 44 different samples simultaneously, saving significant time when a large number of products must be tested.
Here, we demonstrate how the AgraQuant Total Aflatoxin 4/40 ELISA kit can be used together with the SpectraMax® ABS Plus Microplate Reader and SpectraMax® iD5 Multi-Mode Microplate Reader to test for levels of aflatoxins B1, B2, G1, and G2 in one of the most common cannabis products, the flower. A general workflow for sample prep and ELISA is shown in Figure 1.
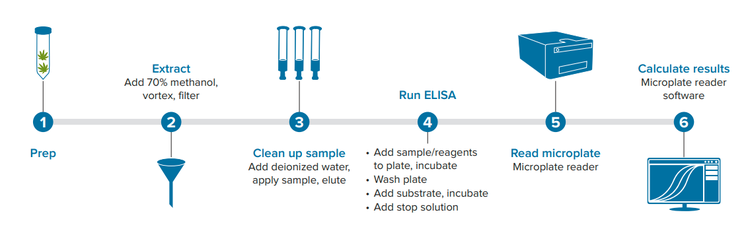
Figure 1. Workflow for cannabis sample processing and ELISA.
Materials
- AgraQuant Total Aflatoxin 4/40 ELISA kit (Romer Labs cat. #10002098)
- AflaStar R Rapid Clean-Up IAC Columns (Romer Labs cat. #10001963)
- Aflatoxin B1 Standard (Sigma cat. #34029)
- Cannabis flower (varieties obtained from California-based dispensary)
- Methanol, HPLC grade (Sigma cat. #34860)
- Ammonium acetate (Sigma cat. # 73594)
- SpectraMax ABS Plus Microplate Reader (Molecular Devices cat. #ABS Plus)
- SpectraMax iD5 Multi-Mode Microplate Reader
Methods
Sample preparation
The cannabis sample was placed in a plastic bag and crushed by hand. One gram of material was weighed and placed in each of four 50 mL conical tubes.
Samples were then spiked with aflatoxin B1 standard at 0, 10, 20, and 30 ppb. 20 mL of 70% methanol was added to the samples, and tubes were vortexed vigorously for three minutes. The resulting extract was filtered through Whatman grade 1 filter paper into a clean 50-mL conical tube.
From each extracted, filtered sample, 4 mL was pipetted into a clean conical tube, and 20 mL deionized water was added. The entire 24 mL was passed through an immunoaffinity chromatography (IAC) column. Aflatoxin in the sample was selectively bound by antibodies in the column. Unbound material was washed away by rinsing the column with 20 mL of 0.5 M ammonium acetate. A clean tube was placed under the column, and sample was eluted with 1 mL of 100% methanol.
Aflatoxin ELISA
Dilution wells for each standard and sample, run in triplicate, were placed in a microwell strip holder. An equal number of antibody-coated microwells was placed in a separate strip holder (ELISA plate). Unused microwells were returned to the foil pouch with desiccant.
100 µL of conjugate was added to each dilution well, followed by 50 µL of aflatoxin standard or spiked sample. A 2:1 ratio of conjugate to sample was thus maintained as directed in the kit protocol. Contents were mixed well with an 8-channel pipettor. 100 µL of each dilution was transferred to the antibody-coated microwells of the ELISA plate, which was then incubated for 15 minutes at room temperature.
After incubation, the ELISA plate was washed five times with deionized water. 100 µL of substrate was added to the plate, and the plate was incubated for five minutes at room temperature. 100 µL of Stop Solution was added, and absorbance of the samples in the plate was measured at 450 nm on the SpectraMax ABS Plus and SpectraMax iD5 readers.
Results
An aflatoxin standard curve consisting of 4, 10, 20, and 40 ppb standards was plotted in SoftMax® Pro Software (Figures 2 and 3). The percent bound, or %B/Bo, for each standard was calculated by the software. B is the mean absorbance value (OD) for each standard and Bo is the mean absorbance for the 0 ppb standard. Wells containing no antigen (Bo) have the maximal absorbance values.

Figure 2. Aflatoxin standard curve generated in SoftMax Pro Software. Aflatoxin standards at 4, 10, 20, and 40 ppb plotted as Logit(B/Bo) vs. Log(Concentration). R2 = 0.997. The standard curve shown was obtained using the SpectraMax ABS Plus reader.
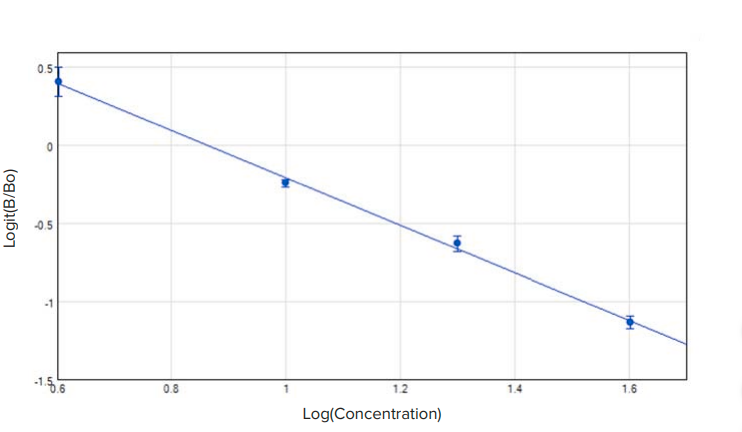
Figure 3. Aflatoxin standard curve, with standards at 4, 10, 20, and 40 ppb plotted as Logit(B/Bo) vs. Log(Concentration. R2 = 0.998. The standard curve shown was obtained using the SpectraMax iD5 Multi-Mode Microplate Reader.
The formula editor and group tables in SoftMax Pro Software were used to calculate %B/Bo and Logit (B/Bo). Once the analysis was set up, it was saved as a SoftMax Pro protocol that could be used to automatically analyze the results of future assays.
Percent recovery of aflatoxin in spiked samples was determined from the standard curve. From 71% to 112% recovery was achieved for samples spiked with 10, 20, and 30 ppb, meeting recommended criteria (Table 1).
% B/B
o
Table 1. Spike recovery.. In samples spiked with aflatoxin B1, percent recovery values fell within acceptable limits for the aflatoxin ELISA.
Conclusion
An ELISA test is easy to use, sensitive, and can handle a large number of test samples compared to other methods like test strips. The AgraQuant Total Aflatoxin 4/40 ELISA kit enables quantitation of aflatoxin at levels required to assure the safety of food and feed products. Here, we have shown that this method can be used to measure aflatoxin recovered from extracts of aflatoxin standard-spiked cannabis flower. Data were obtained using SpectraMax ABS Plus and SpectraMax iD5 readers. Results analyzed using SoftMax Pro Software confirmed that aflatoxin measured in samples was consistent with the amount of aflatoxin spiked into the samples.