
Application Note
High-content assay for morphological characterization of 3D neuronal networks in a microfluidic platform
- Establish a high-throughput 3D neurite outgrowth assay using iPSC-derived neurons
- Generate more in vivo-like results using the microfludic OrganoPlate® platform
- Optimize high-content imaging for evaluation of treatment effects on neuronal networks
Oksana Sirenko | Research Scientist | Molecular Devices
Karlijn Wilschut | Senior Scientist | MIMETAS
Introduction
Establishment of physiologically-relevant in vitro models is crucial to further understanding of the mechanisms of neurological diseases as well as targeted drug development. While iPSC-derived neurons show great promise for compound screening and disease modeling, use of three-dimensional (3D) cultures is emerging as a valid approach for neuronal cell based assay development. 3D cultures are recognized as more closely recapitulating aspects of the human tissues including the architecture, cell organization, as well as cell-cell and cell-matrix interactions1,2.
The focus of the present study was to develop a high-throughput 3D neurite outgrowthassay using iPSCderived neurons developed in the microfluidic OrganoPlate®platform, with the goal of establishing 3D models for neurodegenerative diseases and neurotoxicology screens3. The OrganoPlate®is a high-throughput platform that combines the most recent advances in 3D cell culture, Phaseguides™ and microfluidics4,5,6. The OrganoPlate contains 96 tissue chips suitable for longterm culture of live cells, is amenable for screening purposes, and is compatible with standard laboratory equipment or automated systems, like the ImageXpress®Micro Confocal High-Content Imaging System.
Benefits
- Establish a high-throughput 3D neurite outgrowth assay using iPSC-derived neurons
- Generate more in vivo-like results using the microfludic OrganoPlate ® platform
- Optimize high-content imagingfor evaluation of treatment effects on neuronal networks
Materials
- OrganoPlate ® platform (MIMETAS)
- Human iPSC-derived iCell ® Neurons (Cellular Dynamics International)
- Neuronal media (Cellular Dynamics International)
- Matrigel (Corning)
- Calcein AM (Life Technologies)
- MitoTracker Orange (Life Technologies)
- Hoechst (Life Technologies)
- Saponin (Sigma)
- PBS (Sigma)
- Αντι-β-tubulin III (TUJ-1) antibodies (BD Biosciences)
- ImageXpress Micro ConfocalHigh-Content Imaging System (Molecular Devices)
- MetaXpressHigh-Content Image Acquisition and Analysis Software, version 6.2 (Molecular Devices)
Formation of neurite networks in a 3D matrix
Cell culture, compound treatment, and cell staining steps were performed using the microfluidics assay format. Confocal imaging and analysis protocols were optimized for assessing morphological phenotypes and viability of neurons cultured in a 3D matrix. Human iPSCderived neurons were plated at a density of 30,000 cells/chip after premixing with Matrigel to a final concentration of 7 mg/ml Matrigel in the OrganoPlate®. The Matrigel cell suspension was kept on ice during plating. The seeding volume of this solution varied between 1–1.4 µL per chip depending on cell density and Matrigel concentration. After seeding, the plate was placed into a tissue culture incubator (37°C, 5% CO2) for 30 min to facilitate polymerization of the Matrigel. Next, 50 µL of growth media was added to both the medium inlets and outlets of the chips, and then placed back into the tissue culture incubator. Neurite outgrowthsformed \~24h after plating and extended for up to 14 days in culture.
To evaluate the feasibility of the OrganoPlate platform for testing neurotoxicity and neuronal development, these neuronal cultures were treated with a selection of compounds known to inhibit neurite outgrowth7. Treatment with compounds was performed 24h post plating. Cultures were exposed to treatment for 5 days during which the culture media containing the compounds was changed every second day. Compound dilutions were performed by applying the appropriate final dilution in the media before addition to the medium inlets and outlets of the OrganoPlate chips.
Formation of neurite networks was monitored over time using transmitted light imaging. To assess neuronal viability, cells were stained live using a mixture of three dyes: the viability dye Calcein AM (1 µM), the mitochondria potential dye MitoTracker Orange (1 µM), and the Hoechst nuclear dye (2 µM). The dye mixture was added to the chips and incubated for 60 min followed by replacement with culture medium. Alternatively, cells were fixed in 4% formaldehyde, permeabilized with 0.01% of saponin solution in PBS and stained using fluorophore-conjugated antibodies against β-tubulin III (TUJ-1) neuronal marker (1:100 dilution). Nuclei were stained with Hoechst. Staining of fixed cells with primary antibodies was performed overnight at 4°C.
Phenotypic analysis of 3D cultures
High-content imagingand analysis were utilized for evaluation of treatment effects on neuronal networks. We optimized confocal imaging and analysis protocols for assessing morphology and viability of neurons in this 3D matrix. Images were acquired using ImageXpress MicroConfocal system with 10x, 20x, or 40x objectives. A series of images was acquired at different planes along the focal axis (Z-stack) (Figure 1). A stack of 17-30 planes separated by 3-10 µm was acquired, covering approximately 150–300 µm in depth. All individual images were saved and used for 3D analysis, as well as 2D projection (Maximum Projection or Best Focus) images.

***Figure 1. Neurons in OrganoPlate.*Transmitted light images captured of iCell neurons plated into OrganoPlate capillary wells in Matrigel. Cells were plated at a density of 30,000 cells per chip for 72 hours, then imaged using transmitted light with a 20x, Plan Fluor objective.
Image analysis of Maximum Projection images in 2D
Images were analyzed using MetaXpress®High-Content Image Acquisition and Analysis Software. Automated quantitative analysis of these images was performed using two methods: analysis of projection images (2D) or 3D image analysis on Z-stacks. For the analysis of projection images, Z-stacks of images were converted into 2D maximum projection images at the time of acquisition, then, analyzed using the MetaXpress Neurite Outgrowth application module. Phenotypic readouts included quantitative characterization of the extent and complexity of neural networks via several endpoint measurements including: total neurite outgrowth, number of processes and branches, as well as cell number and viability. Best focus projection images were used for analysis of transmitted light images. Analysis of projection images is rapid, while providing an accurate estimation of the extent of neural networks. However, 2D analysis has its limitations, as this method does not calculate volume measurements and typically counts only a fraction of the objects. Figures 2 and 3 show composite fluorescent images of the control and rotenone treated neuronal cultures with the ‘analysis masks’ used to measure neurite outgrowth. Measurements derived include total neurite outgrowth, number of branches, processes, and the number of total cells (cell bodies). Figure 4 illustrates a decrease of neurite outgrowth length, number of branches and cell viability in response to exposure with neurotoxic compounds including: triphenylphosphate, tetraethyl thiuram, hexachlorophene, rotenone and methyl mercury (10 µM each).

***Figure 2. Live cell staining of neurons.*iCell neurons plated into OrganoPlate capillary wells, in Matrigel, at 30,000 cells per chip. Cells were maintained for 5 days, and then live cells were stained with the viability dye Calcein AM, the mitochondrial integrity dye MitoTracker Orange, and the Hoechst nuclear dye. Then Z-stacks of images in 3 colors were taken using the ImageXpress Micro Confocalsystem in confocal mode using the 60 µm pinhole spinning disc configuration, DAPI, FITC, and TRITC channels, 20x magnification, 25 images 6 µm apart. Maximum projection composite images are shown here for the DAPI and FITC channels.

***Figure 3. 2D analysis of neural networks.*iCell neurons were plated at a density of 30,000 cells per chip for 24 hours, then treated with compounds for 5 days, and then live cells were stained with Calcein AM, MitoTracker Orange, and Hoechst. Z-stacks of images in 3 colors were taken using the ImageXpress Micro Confocal in confocal mode using the 60 µm pinhole spinning disc configuration at 20x magnification. Maximum projection images were analyzed using the MetaXpress Neurite Outgrowth application module. Masks of the cell bodies and neurites are shown in red. Images of control (DMSO) and rotenone (10 mM) treated cells are shown. Disintegration of neuronal connections is detectable as a result of compound treatment.

***Figure 4. 2D measurements characterizing compound effects on neurite networks.*iCell Neurons were cultured as described in Figure 2 and treated with the indicated neurotoxic compounds for 5 days. Z-stacks of images in 3 colors were taken using the ImageXpress Micro Confocalsystem in confocal mode using the 60 spinning disc configuration 20x magnification. Maximum projection images (2D compressions from 3D image stuck) were analyzed using the Neurite Outgrowth application module. Quantitative effects of compounds (10 µM) are shown on the total outgrowth, numbers of live cells, as well as the numbers of branches and processes.
3D visualization and 3D image analysis
MetaXpresssoftware offers a 3D analysis option that allows for combining objects from adjacent Z-planes, as well as 3D visualization of cells and networks. Images were analyzed using a 3D analysis Custom Module using “find fibers” function with which a “fibers” measurement was generated for quantifying neurite outgrowth; cell nuclei were also counted.
Objects are first found in each plane, and then connected in 3D space using the “connect by best match” function. 3D analysis is typically more processingintensive, however delivers improved resolution and quantitation of objects in the 3D space (including multiple overlapping objects). The measurements output from the 3D analysis included the number of neurites (fibers), cell volumes, total volume of fibers, numbers of processes, and branching points. Total numbers of cells (nuclei) were defined by finding “spherical objects” positive for Hoechst stain. Figure 5A shows the analysis masks for the “fibers” and “nuclei”.
An alternate custom module was used for characterization of cell viability (cell bodies positive for Calcein AM) and mitochondrial integrity (cell bodies positive for MitoTracker Orange). Cell scoring analysis in 3D was applied to count and characterize live cells (Calcein AM positive) and cells with intact mitochondria (MitoTracker positive cells). Results calculated via this analysis method included: cell number (total nuclei), live cell count (Calcein AM positive), number of MitoTracker positive cells, as well as cell volumes or fluorescence intensities. Figures 5B and 5C show the analysis masks for the “Calcein AM positive cytoplasm”, “MitoTracker positive cytoplasm” and “nuclei”. Figure 6 presents several measurements characterizing the compound effects on neurite networks: decreases in the numbers of processes (‘fibers” ) and branching points, as well as the numbers of live cells or cells with intact mitochondria.

***Figure 5. 3D analysis of neurite networks.**Cells were treated and stained as described in Figure 2. Z-stacks of images were analyzed using MetaXpress Custom Module Editor 3D analysis option. Two different analysis modules were used for evaluation of complexity of neural networks in 3D: measuring fibers (neurites) and positive cell scoring (marker expression). **(A)**Numbers and volumes of the neurites (fibers), as well as segments and branching points were detected and counted in 3D space using the “find fibers” and “connect by best match” options in the custom module editor (CME). Visualization of fibers (green) and nuclei (blue) are shown. **(B)**The numbers of the total cells, Calcein AM positive “live” cells andvolumes of Calcein AM positive cytoplasm were measured in 3D space using “Cell Scoring” and “Connect by best match” functions in CME. Nuclei shown in blue and Calcein AM positive cytoplasm in green. **(C)*The number of MitoTracker Orange positive cells and the volumes of MitoTracker positive cytoplasm were measured using “Cell Scoring” and “connect by best match” options in CME; nuclei visualized in blue, mitochondria stain is shown in red.

***Figure 6. 3D measurements characterizing compound effects on neurite networks.*iCells Neurons were cultured as described in Figure 2 and treated with indicated neurotoxic compounds for 5 days. The effects of 10 µM concentrations are shown for various measurements from 3D analysis. Numbers and volumes of the neurites (fibers) and branching points were detected and counted in 3D space. In addition, the numbers of the total cells, Calcein AM positive “live” cells, and MitoTracker Orange positive cells were counted and characterized in 3D. Quantitative effects of compounds on the numbers and total volume of neurites (fibers), numbers of branching points the numbers of total, live cells, and MitoTracker Orange positive cells are presented.
Neurotoxicity assay in 3D using OrganoPlates
Phenotypic readouts included quantitative characterization of the extent and complexity of neural networks in 3D by multiplexed measurements. In this neuronal model system, we have evaluated assay reproducibility, characterized multiple measurements, and tested several known neurotoxic compounds. Via these analysis methods, we have accurately measured concentration-dependent inhibitory effects of these compounds on the complexity of neurite networks. Therefore, this proposed method can be used for high-throughput, high-content compound screening for prediction of neurotoxicity.
Conclusion
We have developed a quantitative, confocal high-content imagingmethod that enables high-throughput phenotypic assessment of treatment effects upon the viability and morphology of 3D neuronal cultures using MIMETAS OrganoPlates®. Confocal imaging and multi-parametric 3D analysis allows for counting and characterization of neurites and also provides statistical characterization of neurite development and branching in 3D. Analysis methods enable characterization of neuronal networks and provide quantitative measurements that can be used for defining EC50values and comparing toxic effects of selected compounds.
References
- Chang, TT; Hughes-Fulford, M (2008). Monolayer and Spheroid Culture of Human Liver Hepatocellular Carcinoma Cell Line Cells Demonstrate Distinct Global Gene Expression Patterns and Functional Phenotypes. Tissue Eng Part A. 15(3): 559-567
- Kunz-Schughart, LA et al. (2004). The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol. Screen. 9(4): 273-285
- Trietsch, SJ et al. (2013). Microfluidic titer plate for stratified 3D cell culture. Lab Chip. 13: 3548-3554
- Van Duinen, V et al. (2015). Microfluidic 3D cell culture: from tools to tissue models. Curr. Opin. Biotechnol. 35: 118-26
- Sirenko, O et al. (2014). High-Content HighThroughput Assays for Characterizing the Viability and Morphology of Human iPSCDerived Neuronal Cultures. Assay and Drug Dev. Technologies.12(9-10): 536-47
- Vulto, P et al. (2011). Phaseguides: a paradigm shift in microfluidic priming and emptying. Lab Chip. 11: 1596-1602
- Wevers, NR et al. (2016). High-throughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Sci. Rep. 6: 38856
Learn more about the ImageXpress Micro Confocal High-Content Imaging System >>
Oksana Sirenko| Research Scientist | Molecular Devices
Karlijn Wilschut| Senior Scientist | MIMETAS
前言
建立生理相关的体外模型对于进一步了解神 经疾病的机制以及靶向药物开发至关重要。 iPSC 衍生的神经元显示出对化合物筛选和 疾病建模的巨大希望,然而目前已经出现了 使用三维 (3D) 培养物作为对神经元细胞的 测定开发的有效方法。3D 培养被认为是更 接近人类组织的重演方式,包括结构,细胞 组织,以及细胞 - 细胞和细胞 - 基质相互作 用1,2。
本研究的重点是利用微流控 OrganoPlate® 平台培育的 iPSC 衍生神经元开发高通量 3D 神经突向外生长测定方案,目标是建立神经 退行性疾病和神经毒理学筛查的 3D 模型 3 。 OrganoPlate®是一个高通量平台,结合了 新近的 3D 细胞培养技术,Phaseguides™ 和微流体技术4,5,6。OrganoPlate 包含 96 个 适合长期培养活细胞的组织芯片,适用于筛 选目的,并且与标准实验室设备或自动化系 统兼容,如 ImageXpress®Micro Confocal 高内涵成像系统。
优势
- 使用 iPSC 衍生神经元建立高通 量 3D 神经突向外生长测定
- 使用微流体 OrganoPlate® 平台 生成更多模拟体内结果
- 优化高内涵成像,评估对神经 元网络的治疗效果
材料
- OrganoPlate ® 平台 (MIMETAS)
- 人源 iPS 诱导 iCell® 神经元细胞 (Cellular Dynamics International)
- 神经基质 (Cellular Dynamics International)
- 人工基底膜 (Corning)
- 钙荧光素乙酰氧基甲酯 (Life Technologies)
- MitoTracker Orange (Life Technologies)
- Hoechst (Life Technologies)
- 皂素 (Sigma)
- PBS(Sigma)
- Αντι-β-tubulin III (TUJ-1) 抗体 (BD Biosciences)
- ImageXpress Micro Confocal 高内涵成 像系统 (Molecular Devices)
- MetaXpress 高内涵图像拍摄及分析软 件,Ver 6.2 (Molecular Devices)
3D 模型中神经元网络的形成
使用微流体培养模式进行细胞培养,化合 物处理和细胞染色步骤。对在 3D 基质中 培养的神经元进行共聚焦成像和分析方案 的优化,用于评估形态学表型和活力。与 Matrigel 预混合,使 OrganoPlate® 中 Matrigel 终浓度为 7mg / ml ,然后将人 iPSC 衍生的神经元以 30,000 个细胞/芯片 的密度接种。接种 Matrigel 细胞悬浮液时 保持在冰上操作。该溶液的接种体积在每 片 1-1.4 μL 之间变化,取决于细胞密度和 基质胶浓度。接种后,将板置于组织培养 箱 (37℃,5% CO2 ) 中 30 分钟,以促进基 质胶的聚合。接下来,将 50 μL 生长培养 基添加到芯片的中间入口和出口,然后放 回组织培养箱中。在平板接种后约 24 小时 形成神经突向外生长,并持续培养 14 天。
T为了评估 OrganoPlate 平台测试神经毒性 和神经元发育的可行性,这些神经元培养 物用一系列已知的抑制神经突向外生长的 化合物进行处理 7。在铺板后 24 小时开始 化合物处理。将培养物暴露于外源处理下 5 天,在此期间每隔一天更换含有化合物的 培养基。在添加到 OrganoPlate 芯片的介 质入口和出口之前在介质中施加适当的最 终稀释液来进行化合物稀释。
使用透射光成像随时间监测神经突网络的 形成。为了评估神经元活力,使用三种 染 料的混合物对细胞进行活体染色:活 性染料 Calcein AM (1 μM),线粒体染料 MitoTracker Orange (1 μM) 和 Hoechst 核染料 (2 μM)。将染料混合物加入到芯片 中并孵育 60 分钟,然后用培养基替换。或 者,将细胞固定在 4% 甲醛中,用 0.01% 皂苷溶液在 PBS 中进行穿透,并使用抗 β-微管蛋白III (TUJ-X1) 的神经元标记 物 ( 1:100 稀释 ) 的荧光基团抗体进行染 色。细胞核用 Hoechst 染色。染色固定细 胞时用一抗在 4 ℃ 下孵育过夜。
3D 培养的表型分析
通过高通量的成像和分析来进行对神经网络 的治疗效果的评估。我们优化了共焦成像 和分析方案,在这个 3D 模型内评估神经元 的形态和生存能力。使用 ImageXpress Micro Confocal 系统的 10x,20x,或 40x 的物镜来进行图像拍摄。在焦点轴 ( Z 堆 栈 ) 的不同平面上获得一系列图像 ( 图 1 )。 得到了 3 \~ 10 μm 厚度的 17-30 个面层, 深度约 150 \~ 300 μm。所有单层的图像都 被保存并用于 3D 分析,以及 2D 投影 ( 最 大投影或最佳聚焦 ) 图像。

***图 1 OrganoPlate 中培养的神经元细胞。*在 充满基质胶的 OrganoPlate 培养槽内对接种的 iCell 神经元细胞进行透射光成像。细胞以每芯片 30,000 个细胞的密度培养 72 小时,然后用 20 平 场荧光物镜进行透射光成像
本次获得的 2D 最大投影图像分 析
使用 Meta Xpress® 高通量图像采集和分析 软件对图像进行分析。这些图像的自动 定量分析使用了两种方法:投影图像的分 析 (2D) 或 Z 叠加后的 3D 图像分析。为了 对 投 影 图 像 进 行 分 析 , 在 采 集 时 将 Z-Stack 图像转换为 2D 投影图像,然后使 用 MetaXpress Neurite Outgrowth 应用 模块进行分析。表型读数包括通过几个端 点测量来定量表征神经网络的范围和复杂 性,这些端点测量包括:总神经突起生长、 过程和分支的数量,以及细胞数量和生存 能力。本次得出的最佳聚焦投影图像用于 透射光图像的分析。投影图像的分析是快 速的,同时提供了神经网络的生长范围的 准确估计。然而,二维分析有其局限性, 因为这种方法无法进行体积测量,并且通 常只计算物体的一小部分。图 2 和图 3 显 示了对照组和鱼藤酮处理的神经元培养物 的复合荧光图像,这些神经元培养物具有 用于测量神经突起生长的“分析图层”。 测量结果包括总突起生长,分支数,过程, 总细胞数 ( 细胞体 )。图 4 显示神经突起生 长长度、分支数目和细胞活力随着接触神 经毒性化合物而减少,这些化合物包括: 三苯基磷酸酯、四乙基硫脲、六氯苯基、 鱼藤酮和甲基汞 ( 每种 10 μM )。

图2 神经元活细胞染色 iCell 神经元细胞接种于 OrganoPlate 微孔板的基质胶内,每孔约 30,000 个 细胞。细胞培养 5 天,然后用活性染料 Calcein AM、线粒体完整性染料 MitoTracker Orange 和 Hoechst 核染料对活细胞进行染色。然后使用 ImageXpress Micro Confocal 系统,在共具焦模式下, 使用 60μm 针孔旋转盘结构,DAPI、FITC 和 TRITC 通道,20 倍物镜,以 6 μm 步径拍摄 25 幅图像, 获得了 3 色的 Z 叠加图像。这里给出了本次实验中 DAPI 和 FITC 通道的最大投影合成图像
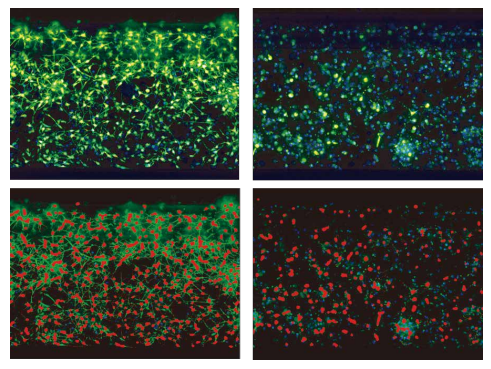
***图 3 神经元网络的 2D 分析。*iCell 神经元以每片 30,000 个细胞的密度培养 24 小时,然后用化合物 处理 5 天,随后用钙调素 AM、MitoTracker Orange 和 Hoechst 染色活细胞。在共具焦模式下,使用 ImageXpress Micro Confocal 系统的 60 μm 针孔旋转圆盘模式,在 20 倍物镜下拍摄了 3 色的 Z 叠加图 像。以上得出的最大投影图像使用 MetaXpress 神经突起生长应用模块进行分析。细胞体和突起的分析 图层用红色标识。图像展示出了对照组图像 (DMSO) 和鱼藤酮 ( 10 毫米 ) 处理的细胞。作为复合治疗的 结果,神经元连接的崩解是可以被检测的

***图 4 特定化合物对神经元网络影响性的 2D 分析。*iCell 神经元如图 2 所示进行培养,并用所指示的神 经毒性化合物处理 5 天。在共聚焦模式下,使用 ImageXpress Micro Confocal 系统,使用 60 个旋转 盘模式、20 倍物镜,拍摄了 3 色的 Z 叠加图像。使用神经突起生长分析模块对本次产生的最大值投影 图像 ( 3D 系列图像的 2D 投影 ) 进行分析。化合物的浓度 (10 μM) 对总生长量、活细胞数、分支数和突 起数有定量影响
3D 可视化和 3D 图像分析
MetaXpress 软件提供了 3D 分析选项,允 许组合来自相邻 Z 平面的对象,以及细胞 和网络的 3D 可视化。使用 3D 分析自定义 模块分析图像,利用“查找纤维”功能生 成用于定量神经突起生长的“纤维”测 量;细胞核也可以用于细胞计数。首先在 每个平面中找到对象,然后使用“最佳连 接”函数在 3D 空间中进行连接。3D 分析 通常是密集处理型的,但是可以提高 3D 空 间中物体 ( 包括多个重叠物体 ) 的分辨率和 数量。从 3D 分析种输出的测量结果包括神 经突 ( 纤维 ) 的数目、细胞体积、纤维总 体积、突起数目和分支点。通过寻找 Hoechst 染色阳性的“球形物体”来确定 细胞总数 ( 细胞核 )。图 5A 显示了“纤 维”和“核”的分析图层。
另一个定制模块用于表征细胞活力 ( Calcein AM 阳性的细胞体 ) 和线粒体完整性 ( MitoTracker Orange阳性的细胞体 ) 的测量。 利用三维细胞评分分析对活细胞 ( Calcein AM 阳性 ) 和线粒体完整细胞 ( MitoTracker 阳性细胞 ) 进行计数和表征。通过该分析 方法计算的结果包括:细胞数 ( 总核 )、活 细胞数 ( 钙调素 AM 阳性 )、MitoTracker 阳性细胞数、以及细胞体积或荧光强度。 图 5B 和 5C 示出了“钙黄绿素 AM 阳性细 胞质”、“MitoTracker 阳性细胞质”和 “细胞核”的分析图层。图 6 给出了几种 表征神经突起网络复合效应的测量值:突 起 (“纤维”) 和分支点的数量以及具有完 整线粒体的活细胞或细胞的数量的减少。

***图 5 神经元网络的 3D 分析。*如图 2 所示方法对细胞进行处理和染色,。使用 MetaXpress 自定义模块 编辑器的 3D 分析选项分析 Z-stacks 图像。用两种不同的分析模块来评估 3D 中神经网络的复杂度:测 量纤维 ( 神经突起 ) 和阳性细胞评分 ( 标记表达 )。(A) 在自定义模块编辑器 (CME) 中使用“查找纤维” 和“通过最佳匹配连接”选项,在 3D 空间中检测和计数神经突 ( 纤维 ) 的数量和体积以及节段和分支 点。显示了纤维 ( 绿色 ) 和核 ( 蓝色 ) 的可视化。(B) 应用"细胞评分"和 CME 的“最佳匹配”功能,在 3D 空间测量总细胞数、钙素 AM 阳性“活”细胞和钙素 AM 阳性细胞质体积。细胞核用蓝色显示,绿 色为阳性细胞质。(C) 在 CME 中使用“细胞评分”和“最佳匹配连接”选项测量 MitoTracker 染色阳 性细胞的数量和 MitoTracker 染色阳性细胞质的体积;细胞核呈蓝色显示,线粒体呈红色

***图 6 特定化合物对神经元网络影响性的 3D 分析。*iCell 神经元如图 2 所示进行培养,并用指示的神经 毒性化合物处理 5 天。10 μm 浓度在三维测量结果中有不同的测量值。在三维空间中检测和计数神经 突起 ( 纤维 ) 和分支点的数量和体积。此外,还计数了细胞总数、钙调素 AM 阳性“活”细 胞和 MitoTracker Orange 阳性细胞,并用 3D 进行表征。给出了化合物对神经突 ( 纤维 ) 的数量和总体 积、分支点数目、总数、活细胞和 MitoTracker Orange 阳性细胞的数量影响
使用 OrganoPlates 进行 3D 神 经毒性分析
表型读数包括通过多重测量定量表征神经 网络在 3D 中的范围和复杂性。在这个神 经元模型系统中,我们评估了试验的重复 性,表征了多次测量,并测试了几种已知 的神经毒性化合物。通过这些分析方法, 我们精确地测量了这些化合物对神经突起 网络复杂性的浓度依赖性抑制作用。因此, 本文提出的方法可用于高通量、高含量化 合物筛选并用于神经毒性的预测。
结论
我们开发了一种定量的、共聚焦的高通 量成像方法,该方法能够使用 MIMETAS OrganoPlates 的神经元培养系统进行高通 量表型评估,表征对 3D 神经元的存活率 和形态的影响。共具焦成像和多参数 3D 分 析允许对神经突进行计数和表征,并且还 提供了神经突发育和分支在 3D 模型中的 统计特征。该分析方法能够对神经元网络 进行表征,并提供可用于确定 EC50 值和比 较所选化合物的毒性效应的定量测量。
参考文献
- Chang, TT; Hughes-Fulford, M (2008). Monolayer and Spheroid Culture of Human Liver Hepatocellular Carcinoma Cell Line Cells Demonstrate Distinct Global Gene Expression Patterns and Functional Phenotypes. Tissue Eng Part A. 15 (3): 559-567
- Kunz-Schughart, LA et al.(2004). The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol. Screen. 9 (4): 273-285
- Trietsch, SJ et al. (2013). Microfluidic titer plate for stratified 3D cell culture. Lab Chip. 13: 3548-3554
- Van Duinen, V et al. (2015). Microfluidic 3D cell culture: from tools to tissue models. Curr. Opin. Biotechnol. 35: 118-26
- Sirenko, O et al. (2014). High-Content High-Throughput Assays for Characterizing the Viability and Morphology of Human iPSC-Derived Neuronal Cultures. Assay and Drug Dev. Technologies.12(9-10): 536-47
- Vulto, P et al. (2011). Phaseguides: a paradigm shift in microfluidic priming and emptying. Lab Chip. 11: 1596-1602
- Wevers, NR et al. (2016). Highthroughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Sci. Rep. 6: 38856
Learn more about the ImageXpress Micro Confocal High-Content Imaging System >>